NLRP3: a sophisticated drug target
NLRP3 (NOD-like receptor pyrin domain-containing protein 3, cryopyrin or NALP3) is the best described inflammasome sensor and an attractive drug target. NLRP3 assembles into a multiprotein inflammasome complex to induce the secretion of IL-1β/IL-18 and pyroptosis in response to infections and cellular damage1,2. However, NLRP3 inflammasome functions can also be detrimental to the host, as its abberant or chronic activation is linked with pathologies such as type-2 diabetes, gouty arthritis, cardiovascular and Alzheimer’s diseases, and rare genetic disorders such as CAPS (cryopyrin-associated-periodic-syndrome)3. Fast-paced research now aims at filling the gaps in the comprehension of NLRP3 inflammasome activation and regulation to develop novel therapeutics.
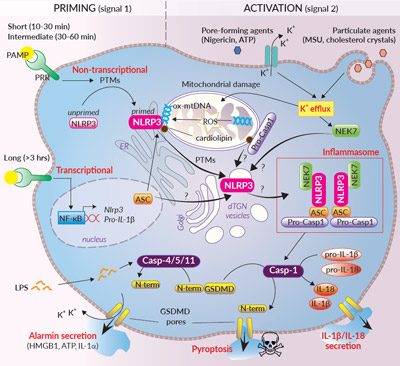
Activation of the NLRP3 inflammasome relies on a 2-signal model. Signal 1 is provided by microbial components or endogenous cytokines and primes NLRP3. Signal 2 is triggered by a plethora of stimuli and promotes NLRP3 activation and inflammasome assembly. This model is much more sophisticated than previously thought. Indeed, different priming mechanisms that depend on the stimulus’ nature and duration have been described. Short and intermediate priming triggers post-translational modifications (PTMs) such as phosphorylation and (de-)ubiquitylation of NLRP3 expressed at basal level. Longer priming triggers NF-κB-mediated transcription of NLRP3, pro-caspase-1, and pro-IL-1β. Primed NLRP3 remains in an auto-repressed state until activation2,4. NLRP3 can be activated by a wide range of stimuli that are structurally and chemically unrelated (e.g. pore-forming toxins, activators of ion channels, uric acid crystals, β-amyloid proteins) suggesting that NLRP3 doesn’t bind directly to these molecules. NLRP3 rather senses downstream cytosolic stress signals such as ion imbalances (e.g. K+ efflux), externalized mitochodrial (mt) cardiolipin and oxidized mtDNA, the two latter being proposed as NLRP3 ligands2-5. Yet, the exact mechanisms underlying NRLP3 activation remain a controversial topic4,6.
Two major NLRP3 inflammasome responses, canonical and non-canonical, have been described and differ in their caspase (Casp) recruitment1,2,6. Upon activation, the canonical response is driven by aggregation of NLRP3 with the ASC adaptor and pro-Casp-1 (Fig. 2 inside). Self-cleaved active Casp-1 induces the maturation of pro-IL-1β/pro-IL-18, and cleavage of the pore-forming gasdermin D (GSDMD), leading to secretion of IL-1β/-18 and pyroptosis1,6. The non-canonical response relies on the activation of Casp-4/5 (human) or Casp-11 (mouse) upon the sensing of cytosolic LPS (Fig. 2 inside). Casp-4/5/11 cannot cleave pro-IL-1β/-18, but they trigger stress signals through GSDMD pore formation at the plasma membrane. Thus, this ultimately induces NLRP3 inflammasome assembly and Casp-1-mediated IL-1β/-18 maturation and secretion1,6.
Organelles play a key role in NLRP3 inflammasome assembly2,4-6. Coordinated priming and activation signals seem to orchestrate trafficking of NLRP3 and other proteins to mitochondria, endoplasmic reticulum (ER), and Golgi. NEK7 (NIMA-related protein kinase 7) was recently identified as an integral component of the NLRP3 inflammasome, but timing and location for its association with NLRP3 is not fully elucidated yet. While some studies suggest that the first steps of NLRP3 inflammasome assembly occurs at mitochondria-associated ER membranes (MAMs), a recent report indicates that activation and inflammasome assembly rather occur around dispersed trans-Gogli network (dTGN) vesicles, without mitochondrial trafficking7. Whether multiple organelle-linked mechanisms can rule NLRP3 inflammasome assembly requires further work.
Intricate activating and inhibiting PTMs of NLRP3 inflammasome components have emerged as important regulatory means during priming and activation steps2,4,6. Research on the nature, timing and location of regulatory PTMs will help in identifying new targets for fine-tuned therapeutics with minimal side-effect to replace existing NLRP3 inhibitors and IL-1β/-18 blockade strategies3,4.
References:
1. Broz P. and Dixit V.M., 2016. Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol. 16:407.
2. Groslambert M. & Py B. 2018. Spotlight on the NLRP3 inflammasome pathway. J. Inflamm. Res. 11:359.
3. Mangan M.S.J. et al., 2018. Targeting the NLRP3 inflammasome in inflammatory diseases. Nat. Rev. Drug. Discov. 17:558.
4. Swanson K.V. et al., 2019. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 19:477.
5. Hamilton C. & Anand P.K. 2019. Right place, right time: localisation and assembly of the NLRP3 inflammasome. F1000Research. 8:676.
6. Yang Y., et al., 2019. Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis. 10:128.
7. Chen J. & Chen Z.J., 2018. PtdIns4P on dispersed trans-Golgi network mediates NLRP3 inflammasome activation. Nature. 564:71.