Immunoglobulin G
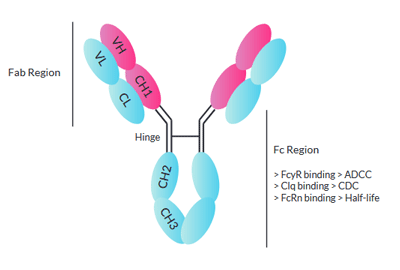
Immunoglobulin G (IgG) antibodies are large molecules
composed of two heavy chains γ and two light chains, either κ or λ.
They can be separated into two regions: the Fab (fragment-antigen binding) that contains the variable domain responsible for the antibody specificity, and the Fc (fragment crystalline) that binds specific proteins to induce immune responses such as opsonization and cell lysis.
The IgG class is divided into four isotypes: IgG1, IgG2, IgG3 and IgG4 in humans, and IgG1, IgG2a, IgG2b and IgG3 in mice. They share more than 95% homology in the amino acid sequences of the Fc regions but show major differences in the amino acid composition and structure of the hinge region.
The Fc region mediates effector functions, such as antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC).
In ADCC, the Fc region of an antibody binds to Fc receptors (FcγRs) on the surface of immune effector cells such as natural killers and macrophages, leading to phagocytosis or lysis of the targeted cells. In CDC, the antibodies kill the targeted cells by triggering the complement cascade at the cell surface. IgG isoforms exert different levels of effector functions increasing in the order of IgG4 < IgG2 < IgG1 ≤ IgG3.
Antibody Effector Activities and Affinities
| Isotype | Species | ADCC | CDC | Protein A binding | Protein G binding | Protein L binding |
|---|---|---|---|---|---|---|
| IgG1 | Human | +++ | +++ | ++++ | ++++ | ++++ |
| IgG2 | Human | +/- | + | ++++ | ++++ | ++++ |
| IgG3 | Human | +++ | ++++ | - | ++++ | ++++ |
| IgG4 | Human | +/- | - | ++++ | ++++ | ++++ |
| IgG1 | Mouse | - | +/- | + | ++++ | ++++ |
| IgG2a | Mouse | +++ | +++ | ++++ | ++++ | ++++ |
| IgG2b | Mouse | +++ | +++ | +++ | +++ | ++++ |
| IgG3 | Mouse | +++ | + | ++ | +++ | ++++ |
| IgG | Rabbit | N/A | +++ | ++++ | +++ | + |
| IgG2b | Rat | ++ | ++ | - | ++ | ++++ |



