TLR3: racing for vaccine advantages
Toll-like receptors (TLRs) play a pivotal role in the initiation of prompt innate immune defenses, as well as in the activation of adaptive immune cells for enhanced and memory responses. Thus, TLR agonists are attractive candidates for vaccine adjuvants and cancer therapeutics. Yet, major challenges limit their translation from bench to bedside. As of today, hundreds of TLR ligands have been identified, but only one TLR4 agonist (MPL®, monophosphoryl lipid A) and one TLR9 agonist (CpG 1018®) have been approved for anti-infectious vaccine adjuvantation1,2. One TLR7 agonist (Imiquimod) is currently used as a stand-alone anti-viral and anti-cancerous drug1,2. Excitingly, recent advances might now bring TLR3 under the spotlight.
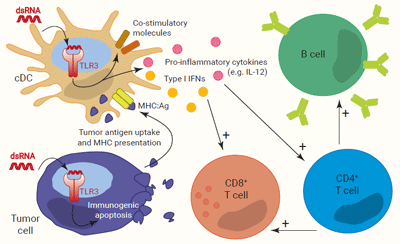
TLR3 is expressed by immune cells, mostly conventional dendritic cells (cDCs), and non-immune cells, including transformed cells, as described in several epithelial cancers3,4. It senses intracellular double-stranded (ds)RNA which signs the presence of a virus as well as damaged cells3-5. TLR3 and dsRNA interaction is independent of the RNA sequence, but its length must be at least 40 bp for TLR3 dimerization and signaling3,5.
Akin to TLR4, TLR3 triggering induces the simultaneous activation of IRF and NF-κB. In cDCs, it results in the upregulation of co-stimulatory molecules and the production of both type I IFNs and pro-inflammatory cytokines. Of note, IFN-α/β and IL-12 are critical for CD8+ and Th1-biased CD4+ T cell activation, respectively. CD4+ Th1 cells, in turn, potentiate B cell activation and differentiation into plasmocytes6-8. Moreover, in tumors, TLR3 is reported to induce immunogenic apoptosis in cancer cells, while sparing normal cells. This allows effective uptake and MHC presentation of tumor-associated antigens by cDCs to specific CD4+ and CD8+ T cells4.
Altogether, TLR3 expression and signaling pathways in normal and malignant cells make it a one-of-a-kind target for the development of prophylactic anti-infectious and therapeutic anti-tumoral vaccine adjuvants
At least four criteria determine the access of TLR3 agonists to the restrictive circle of vaccine adjuvants approved by regulatory agencies:
- 1) compliance with current Good manufacturing Practices (cGMP),
- 2) tolerability and absence of major side effects,
- 3) potency to enhance antibody responses, as well as CD4+ and CD8+ T cells immunity, and
-
4) contribution to the generation of memory cells.
Poly(I:C) (polyinosinic polycytidylic acid), a mix of imperfectly annealed synthetic 1,5 to 8 kb dsRNAs and ssRNAs, was the first candidate for TLR3-targeted adjuvantation1
It was reported to efficiently activate DCs and potentiate antigen-specific T cell and B cell proliferation in preclinical studies1,9. However, it was also reported to have toxic side effects in humans, probably conferred by its undefined chemical structure and poor homogeneity2,7,8. Therefore, more stable and/or safer derivatives of Poly(I:C) were developed, and entered clinical trials. For example, Poly-ICLC, Poly-IC12U, and PIKA were included in vaccination strategies against HIV, Influenza, Rabies lyssavirus, and more recently, SARS-CoV-27. Regarding cancer treatments, Poly-ICLC and Poly-IC12U have been tested in combination with other therapies in a range of solid tumors2,7,8. To date, these molecules have reached phase I or phase II, and closed trials are awaiting results2,7,8. Importantly, they still present a lack of homogeneity due to technical limitations in the manufacturing process.
Continual efforts to find potent and lot-to-lot reproducible TLR3 agonists have led to the development of two new promising adjuvants.
NexaVant™ is a synthetic, cGMP grade, 424 bp dsRNA generated through in vitro transcription of a viral nucleotide segment10. It exhibits extreme homogeneity and absence of serious toxicity in preclinical studies10. It is a potent inducer of both IRF and NF-κB activation in cellular assays10. Moreover, it promotes effective DC activation and migration into draining lymph nodes, as well as antigen-specific B, CD4+ Th1 and CD8+ T cell responses in mouse immunization models10. TL-532 is another homogenous synthetic dsRNA of 70 bp length11. It is safe in non-human primates, and triggers activation of the immune system with anti-cancer effects in mice11,12.
Different delivery platforms, such as aluminum salts, oil/water emulsions, lipids, or polymer nanoparticles, are available and allow prolonged bioavailability of immunostimulants together with specific antigens2. These formulations aim at delivering the lowest possible doses of adjuvant to elicit robust immune responses and avoid systemic side-effects. The next challenge is now to find the best formulations for the new TLR3 agonists, depending on the vaccination intended responses.
References :
Ong, G.H., et al., 2021. Front Cell Infect Microbiol, 2021. 11: p. 745016.
Zhao, T., et al., 2023. Signal Transduct Target Ther, 8(1): p. 283.
Chen, Y.G. and S. Hur, 2022. Nat Rev Mol Cell Biol, 23(4): p. 286.
Estornes, Y., et al., 2017. TRAIL, Fas Ligand, TNF and TLR3 in Cancer, Resistance to Targeted Anti-Cancer Therapeutics, p159-185.
Kawai, T. and S. Akira, 2008. Annals of the New York Academy of Sciences, 1143(1): p. 1-20.
Komal, A., et al., 2021. Immunol Res. 69(4): p. 312-322.
Yang, J.-X., et al., 2022 Pharmaceutics, 14(2): p. 423.
Yang, Y., et al., 2022 Front Immunol,13:1049340.
Robinson, R.A., et al., 1976. JNCI: Journal of the National Cancer Institute, 57(3): 599.
Ko, K.H., et al., 2023. Front Immunol, 14:1075291.
Thierry, S., et al., 2023. Microb Cell. 10(6): p. 117-132.
Le Naour, J., et al., 2023. Oncoimmunology, 12(1): p. 2227510.