Human TGF-β Antibody - Fresolimumab Biosimilar
| Product | Unit size | Cat. code | Docs. | Qty. | Price | |
|---|---|---|---|---|---|---|
|
Anti-hTGF-β-hIgG4 (S228P) Human TGF-β1/β2/β3 (Fresolimumab) antibody - Human IgG4 (S228P) |
Show product |
100 µg 3 x 100 µg |
htgfb-mab14
|
|
Anti-human TGF-β1, β2, β3- Fresolimumab biosimilar - CAS #948564-73-6
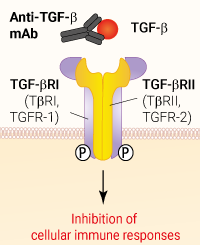
Binding of Anti-TGF-β Fresolimumab
InvivoGen also offers:
Anti-hTGF-β-hIgG4 (S228P) is a biosimilar antibody of Fresolimumab, a human transforming growth factor beta (TGF-β) antibody that blocks all mammalian active subtypes of TGF-β: TGF-β1, TGF-β2, and TGF-β3. This monoclonal antibody (mAb) is under investigation for the treatment of pulmonary fibrosis and various cancer types (non-small cell lung cancer).
Anti-hTGF-β-hIgG4 (S228P) comprises the variable region of Fresolimumab and the human IgG4 (S228P) constant region of Fresolimumab for low/no effector functions.
This antibody can be used together with HEK-Blue™ TGF-β cells for screening and neutralization assays to block TGF-β signaling induced by recombinant human TGF-β (see figure).
Key features
- Each lot is functionally tested and validated.
- The complete sequence of the antibody construct has been verified.
- The absence of endotoxins is determined by the EndotoxDetect™ assay.
All InvivoGen products are for internal research use only, and not for human or veterinary use.
Back to the topSpecifications
Application: Neutralization assay, ELISA
Isotype: Human IgG4 (S228P), kappa
Recommended isotype control: Human IgG4 (S228P)
Target: Human (h)TGF-β1, hTGF-β2, hTGF-β3
Species reactivity: Human
Clone: Fresolimumab, GC1008
Cas number: 948564-73-6
Sterility: 0.2 µm filtration
Source: CHO cells
Production: Animal-free
Purification: Protein A
Molecular weight: 144.4 kDa
Physical form: Lyophilized
Formulation buffer: Sodium phosphate buffer with glycine, saccharose, and stabilizing agents
Preservative: Azide-free
Reconstitution buffer: Sterile water (not provided)
Purity: ≥ 95 %
Quality control: Each lot is functionally tested and validated
Back to the topContents
Anti-hTGF-β-hIgG4 (S228P) purified monoclonal antibody is provided azide-free and lyophilized. It is available in two quantities:
- htgfb-mab14: 100 µg
- htgfb-mab14-03: 3 x 100 µg
![]() The product is shipped at room temperature.
The product is shipped at room temperature.
![]() Upon receipt, store lyophilized antibody at -20 °C.
Upon receipt, store lyophilized antibody at -20 °C.
![]() Lyophilized product is stable for at least 1 year.
Lyophilized product is stable for at least 1 year.
![]() Avoid repeated freeze-thaw cycles.
Avoid repeated freeze-thaw cycles.
Details
Fresolimumab background
Fresolimumab is a fully human IgG4 (S228P) monoclonal antibody (mAb) designed to target the transforming growth factor beta (TGF-β) [1]. As a pan-specific mAb, it binds and neutralizes all three active mammalian isoforms of TGF-β: TGF-β1, TGF-β2, and TGF-β3 [1].
TGF-β is a multifunctional cytokine that regulates critical cellular processes such as proliferation, apoptosis, differentiation, and migration [2,3]. Dysregulated TGF-β signaling is implicated in several fibrotic diseases (e.g., idiopathic pulmonary fibrosis (IPF), chronic obstructive pulmonary disease (COPD)) and plays a role in cancer development and progression [1].
Fresolimumab (GC-1008) has been shown to inhibit the pathological effects of excessive TGF-β activity and was developed as a potential treatment for IPF, melanoma, renal cell carcinoma, and focal segmental glomerulosclerosis [1]. Currently, it remains under clinical investigation for its potential efficacy in osteogenesis imperfecta and non-small cell lung cancer (NSCLC) [4].
References:
1. Grütter C, et al., 2008. A cytokine-neutralizing antibody as a structural mimetic of 2 receptor interactions. Proc Natl Acad Sci U S A. 105(51):20251-6.
2. Travis MA. & Sheppard D., 2014. TGF-β activation and function in immunity. Annu Rev Immunol. 32:51-82.
3. Taylor AW., 2009. Review of the activation of TGF-beta in immunity. J Leukoc Biol. 85(1):29-33.
4. https://www.pharmaceutical-technology.com/data-insights/fresolimumab-sanofi-non-small-cell-lung-cancer-likelihood-of-approval/?cf-view






