FLA-ST (Standard or Ultrapure)
| Product | Unit size | Cat. code | Docs. | Qty. | Price | |
|---|---|---|---|---|---|---|
|
FLA-ST TLR5 Agonist - Flagellin from S. typhimurium |
Show product |
100 µg |
tlrl-stfla
|
|
||
|
FLA-ST Ultrapure TLR5 Agonist - Ultrapure flagellin from S. typhimurium |
Show product |
50 µg 10 µg |
tlrl-epstfla-5
|
|
Purified Flagellin from S. typhimurium
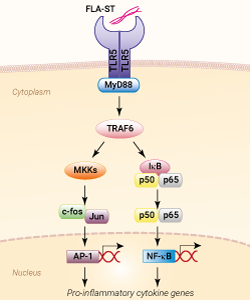
FLA-ST is flagellin isolated from the Gram-negative bacteria Salmonella typhimurium. Flagellin is the principal component of the flagella present in many Gram-negative and Gram-positive bacteria. It is a proinflammatory molecule recognized by distinct types of pattern recognition receptors (PRRs); the surface-localized Toll-like receptor 5 (TLR5) [1] and the cytosolic NOD-like receptors (NLRs), NLRC4 and NAIP5 [2].
Mode of action:
Extracellular flagellin is detected by TLR5 resulting in MyD88-mediated NF-κB activation, cytokine, and nitric oxide production depending on the nature of the TLR5 signaling complex [3].
Intracellular flagellin is detected by NLRC4 (also known as IPAF) and NAIP5. Recognition by NLRC4 and NAIP5, leads to inflammasome assembly, triggering caspase-1 activation of IL-1β and IL-18.
InvivoGen provides FLA-ST with two grades of purity:
- FLA-ST was purified by acid hydrolysis, heating, and ultrafiltration. The purity of FLA-ST is estimated at 10%.
- FLA-ST Ultrapure was obtained following an additional purification step using monoclonal anti-FliC affinity chromatography. The purity of FLA-ST ultrapure is >95%.
References:
1. Hayashi F. et al., 2001. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 410(6832):1099-103.
2. Zhao Y. et al., 2011. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature 477(7366):596-600.
3. Mizel S.B. et al., 2003. Induction of macrophage nitric oxide production by Gram-negative flagellin involves signaling via heteromeric Toll-like receptor 5/Toll-like receptor 4 complexes. J Immunol. 170(12):6217-23.
Specifications
FLA-ST
Specificity: TLR5 agonist
Working concentration: 10 ng - 10 µg/ml
Solubility: 0.5 mg/ml in water
Quality Control
- Purity: ≈ 10%
- Endotoxin levels: 1-10 EU/µg
- Activation of TLR5 has been confirmed using HEK‑Blue™ TLR5 cells.
FLA-ST Ultrapure
Specificity: TLR5 agonist
Working concentration: 10-100 ng/ml
Solubility: 0.5 mg/ml in water
Quality Control
- Purity: > 95%
- Endotoxin levels: <0.05 EU/µg
- Activation of TLR5 has been confirmed using HEK‑Blue™ TLR5 cells.
Contents
FLA-ST
- 100 µg FLA-ST (standard flagellin from S. typhimurium)
- 1.5 ml endotoxin-free water
FLA-ST Ultrapure
FLA-ST Ultrapure (purified flagellin from S. typhimurium) is available in two pack sizes:
- tlrl-epstfla: 10 µg
- tlrl-epstfla-5: 50 µg
- 1.5 ml endotoxin-free water
![]() FLA-ST and FLA-ST Ultrapure are shipped at room temperature
FLA-ST and FLA-ST Ultrapure are shipped at room temperature
![]() Upon receipt, store at -20°C.
Upon receipt, store at -20°C.
Details
Toll-like receptor 5 (TLR5)
The Toll-like receptor 5 (TLR5) is an important pattern recognition receptor (PRR) of the innate immune system playing an essential role in the respiratory tract, gastrointestinal tract, and liver. It recognizes a wide variety of pathogen-associated molecular patterns (PAMPs), specifically flagellin, the major structural protein of Gram-positive and Gram-negative bacterial flagella [1]. TLR5 is expressed constitutively in epithelial cells and immune cells, such as monocytes and immature dendritic cells (DCs). It is preferentially found on the apical side of respiratory epithelia in both mice and humans [1]. Activation of the receptor stimulates the production of proinflammatory cytokines, such as TNF-α, through signaling via the adaptor protein MyD88 [1-3]. TLR5 can generate a pro-inflammatory signal as a homodimer suggesting that it might be the only TLR participating in flagellin recognition [3]. However, TLR5 may require the presence of a co-receptor or adaptor molecule for efficient ligand recognition and/or signaling [4].
NLRC4 inflammasome
Inflammasomes are cytoplasmic multi-protein complexes, characterized by a primary sensor, that assemble in response to infections and cellular damage. NLRC4 is an indirect sensor that must associate with NAIP (NLR family apoptosis inhibitory protein) to induce the assembly of an NLRC4 inflammasome. A single NAIP operates upstream of NLRC4 in humans and recognizes intracellular Flagellin, Needle, or Rod from several Gram-negative bacterial strains [5]. These ligands are sensed by NAIP upon bacterial invasion, or cytosolic translocation through the bacterial type III or IV secretion systems (T3SS or T4SS). Once recruited by NAIP, NLRC4 triggers a homo-polymerization through NBD-NBD interactions, allowing a CARD clustering [5]. The NLRC4 polymer further associates to pro-caspase-1, either through direct CARD-CARD interaction or through the binding of the ASC (apoptosis-associated speck-like protein) adaptor [1]. Activation of caspase-1 induces the maturation of pro-IL-1β/pro-IL-18, cleavage of the pore-forming gasdermin D (GSDMD), secretion of IL-1β/-18, and pyroptosis [5-7]. The NLRC4 inflammasome appears to protect mucosal barriers such as the lung, stomach, and intestine from invading bacteria 8]. Gain-of-function mutations have been described in human NLRC4 and are associated with auto-inflammatory conditions [7].
References
1. Yang J. & Yan H. 2017. TLR5: beyond the recognition of flagellin.Cell Mol Immunol. 14(12):1017-1019.
2. Gewirtz AT. et al., 2001. Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J Immunol, 167(4):1882-5.
3. Hayashi F. et al., 2001. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature, 410(6832):1099-103.
4. Tallant T. et al., 2004. Flagellin acting via TLR5 is the major activator of key signaling pathways leading to NF-kappa B and proinflammatory gene program activation in intestinal epithelial cells. BMC Microbiol. 4(1):33.
5. Zhang L. et al., 2015. Cryo-EM structure of the activated NAIP2-NLRC4 inflammasome reveals nucleated polymerization. Science. 350:404-409.
6. Zhao Y. et al., 2011. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature. 477: 596-600.
7. Bauer R. & Rauch I., 2011. The NAIP/NLRC4 inflammasome in infection and pathology. Mol. Aspects Med. 2020 Jun 1:100863.