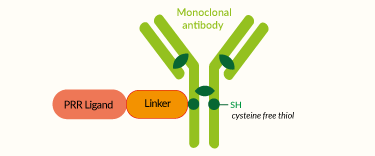
Antibody Drug Conjugates (ADCs)
Antibody-drug conjugates (ADCs) are an onco-therapeutic class of bioconjugates designed for targeted payload delivery to cancer cells or cells of the tumor microenvironment (TME) [1]. Such platforms allow for reaching an enhanced therapeutic index and minimal off-target toxicity when compared to the administration of free payloads [2]. A number of ADCs featuring different cytotoxic cargos have been granted FDA approval for the treatment of solid and hematologic malignancies [2, 3].
Overall ADC structure
All ADCs are constituted by three key elements [1, 4]:
- a monoclonal antibody (mAb) specific for a cognate antigen, selected for high and homogenous surface expression in tumor cells or other cells of TME.
- a payload/cargo consisting of either a highly cytotoxic drug or a potent immunostimulant, which in their free form display limited tolerability.
- a chemical linker that connects the payload to the mAb, preventing its premature systemic release while permitting its efficient delivery to target cells.
Immunostimulatory ADCs
ADCs featuring PRR agonist cargos have been designed to target cancer cells and potentiate immune responses in the TME by triggering the production of pro-inflammatory cytokines and chemokines. This class of ADCs is also named immunostimulatory ADCs, immune-stimulating ADCs, or Immune-stimulating antibody conjugates (ISACs). These types of bioconjugates represent promising avenues for cancer immunotherapy. They have demonstrated robust activity as monotherapy or in combination therapies in pre-clinical studies and are now entering clinical trials [5-8].
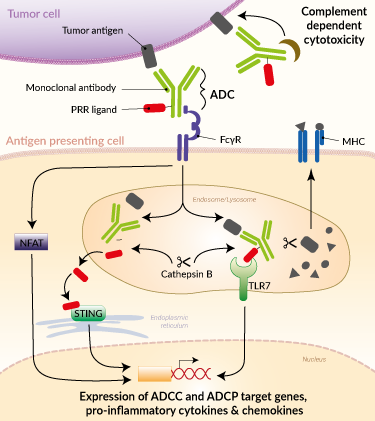
Immunostimulatory ADC-mediated immune responses
Mechanisms of action of immunostimulatory ADCs
Immune-stimulating ADCs potentiate anti-tumor immune responses by (see illustration):
- Tumor cell killing through antibody-mediated effector functions upon specific recognition of tumor antigen by the mAb variable region and binding of the mAb Fc fragment by FcγR-expressing cells, such as antigen-presenting cells (activation of NFAT-mediated ADCC and ADCP), or by the complement ('complement-dependent cytotoxicity),
- PRR activation (e.g. STING or TLR7) in the tumor target cells (not shown) or neighboring myeloid cells after ADC internalization.
These two events act in synergy to induce the secretion of pro-inflammatory cytokines, recruitment of immune cells to the tumor site, and antigen presentation to tumor-specific T cells [5, 9].
InvivoGen offers biological tools for building immunostimulatory ADCs:
- Conjugatable PRR ligands: TLR7 and STING agonists
- Anti-HER2-hIgG1: a monoclonal human IgG1 antibody against HER2, one of the prototypical target antigens for ADCs
-
Anti-TROP2-hIgG1: a monoclonal human IgG1 antibody against TROP2, one of the prototypical target antigens for ADCs
![]() Learn more about bioconjugation
Learn more about bioconjugation
References:
1. Gingrich J., 2020. How the Next Generation Antibody Drug Conjugates Expands Beyond Cytotoxic Payloads for Cancer Therapy – ADC Review / Journal of Antibody-drug Conjugates. DOI: 10.14229/jadc.2020.04.07.001.
2. Drago J.Z. et al., 2021. Unlocking the potential of antibody–drug conjugates for cancer therapy. Nat Rev Clin Oncol. 18-6:327.
3. Tarantino P. et al., 2022. Antibody-drug conjugates: smart chemotherapy delivery across tumor histologies. CA Cancer J Clin. 72:165-182.
4. Fuentes-Antras J. et al., 2023. Antibody-drug conjugates: in search of partners of choice. Trends in Cancer. 9(4):339-348.
5. Ackerman S.E. et al., 2021. Immune-stimulating antibody conjugates elicit robust myeloid activation and durable antitumor immunity. Nat. Cancer. 2(1):18.
6. https://clinicaltrials.gov/ct2/show/NCT04278144
7. https://clinicaltrials.gov/ct2/show/NCT04460456
8. https://www.mersana.com/pipeline/overview/
9. Demaria O. & Vivier E., 2020. ISACs take a Toll on tumors. Nat Cancer. 2(1):12.