Recombinant anti-mouse CTLA4 antibody
| Product | Unit size | Cat. code | Docs. | Qty. | Price | |
|---|---|---|---|---|---|---|
|
Anti-mCTLA4-mIgG2a InvivoFit™ 9D9-derived mouse monoclonal antibody against murine CTLA-4 |
Show product |
1 mg 10 mg 50 mg 100 mg |
mctla4-mab10-1
|
Recombinant mouse CTLA-4 antibody for in vivo use
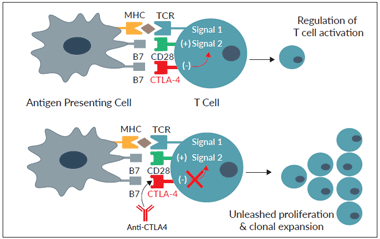
Effect of Anti-CTLA4 on T cell activation
Anti-mCTLA4-mIgG2a InvivoFit™ is a mouse anti-mouse monoclonal antibody (mAb) featuring the variable region of the previously described anti-mCTLA-4 clone 9D9 [1] and a murine IgG2a constant region. Its sequence is 100% murine (constant and variable regions), ensuring minimal immunogenicity upon repeated injections into mice. This mAb targets murine cytotoxic T-lymphocyte-associated protein 4 (mCTLA-4) and blocks CTLA4-mediated negative signals downregulating T cell activation. Thus, anti-mCTLA-4 mAb unleashes T cell activity [1, 2].
Notably, the 9D9 mAb, along with the most commercially available anti-mCTLA4 mAbs feature the IgG2b isotype which displays low antitumor activity in vivo [2]. To overcome this issue, Anti-mCTLA4-mIgG2a InvivoFit™ was generated by recombinant technology and features the murine IgG2a isotype, which mediates potent cytotoxic functions. Anti-mCTLA4-mIgG2a is provided in an InvivoFit™ grade, a high-quality standard specifically adapted to in vivo studies.
Key features of Anti-mCTLA4-mIgG2a InvivoFit™:
- Sequence is 100% murine
- Features the IgG2a isotype (associated with high antitumor activity)
- Filter-sterilized (0.2 µm), endotoxin level < 1 EU/mg
- Specifically designed for in vivo studies in mice
- Low aggregation < 5%
- Produced in animal-free facilities and defined media
Anti-mCTLA4-mIgG2a InvivoFit™ is produced in Chinese hamster ovary (CHO) cells, purified by affinity chromatography with protein A, and its binding to mCTLA-4 is validated using ELISA.
This antibody is also available in bulk quantity, please contact us
References:
1. Selby MJ. et al., 2013. Anti-CTLA-4 antibodies of IgG2a isotype enhance antitumor activity through reduction of intratumoral regulatory T cells. Cancer Immunol Res. 1(1):32-42.
2. Ribas A. & Wolchock J.D., 2018. Cancer immunotherapy using checkpoint blockade. Science. 359:1350-55.
Specifications
Specificity: Targets cells expressing murine CTLA-4
Formulation: Lyophilized from 0.2 μm filtered solution in 150 mM sodium chloride, 20 mM sodium phosphate buffer with 5% saccharose
Clonality: Monoclonal antibody
Isotype: Murine IgG2a, kappa
Control: mIgG2a InvivoFit™ isotype control
Clone: 9D9
Source: CHO cells
Purity: Purified by affinity chromatography with protein A
Tested application: ELISA
Quality control:
- Binding confirmed using ELISA
- The complete sequence of this antibody has been verified
- < 5% aggregates (confirmed by size exclusion chromatography)
- Endotoxin level <1 EU/mg (determined by the LAL assay)
Contents
Anti-mCTLA4-mIgG2a InvivoFit™ is filter-sterilized (0.2 µm), endotoxin-free, azide-free, and lyophilized.
This product is available in four pack sizes:
- mctla4-mab10-1: 1 mg
- mctla4-mab10-10: 10 mg
- mctla4-mab10-50: 50 mg (5 x 10 mg)
- mctla4-mab10-100: 100 mg (10 x 10 mg)
![]() The product is shipped at room temperature.
The product is shipped at room temperature.
![]() Store lyophilized antibody at -20 °C.
Store lyophilized antibody at -20 °C.
![]() Lyophilized product is stable for at least 1 year
Lyophilized product is stable for at least 1 year
![]() Avoid repeated freeze-thaw cycles.
Avoid repeated freeze-thaw cycles.
InvivoFit™
InvivoFit™ is a high-quality standard specifically adapted for in vivo studies. InvivoFit™ products are filter-sterilized (0.2 µm) and filled under strict aseptic conditions in a clean room. The level of bacterial contaminants (endotoxins and lipoproteins) in each lot is verified using a LAL assay and a TLR2 and TLR4 reporter assay.
Back to the topDetails
The cytotoxic T-lymphocyte-associated protein 4 (CTLA-4, also known as CD152) is an inhibitory receptor expressed on activated T lymphocytes. T-cell activation requires two signals. Signal 1 is delivered upon interaction of the T cell receptor (TCR) with antigenic peptides bound to major histocompatibility complex (MHC) molecules on antigen-presenting cells (APCs). Signal 2 is delivered upon interaction of the co-stimulatory receptor CD28 with the B7 family ligands, B7-1 (CD80) and B7-2 (CD86), on APCs.
CTLA-4 is an immune checkpoint molecule upregulated upon T cell activation. It exerts competitive binding to CD28 ligands, thereby preventing T-cell overstimulation. CTLA-4 blockade with monoclonal antibodies (mAbs) has been used to treat patients with metastatic melanoma.
In vivo Data
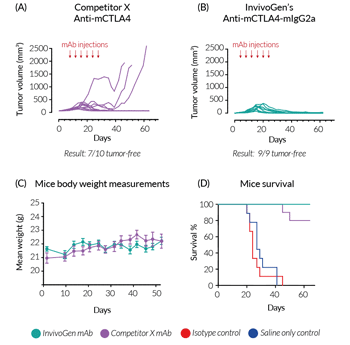
In vivo efficacy of InvivoGen’s Anti-mCTLA4-mIgG2a
In vivo efficacy of InvivoGen’s Anti-mCTLA4-mIgG2a
Unlike most immune checkpoint (IC) mAbs on the market, which are produced in hybridomas, InvivoGen’s mouse anti-mouse mAbs are expressed in virus-free CHO cells. This ensures structural stability as well as guaranteeing the highest purity and batch-to-batch consistency; important for mAb efficacy in vivo.
BALB/c mice were challenged with the murine colorectal carcinoma cell line, CT26. After 8 days, treatment was started with InvivoGen's Anti‑mCTLA4‑mIgG2a (recombinant mAb), Competitor X’s Anti‑mCTLA4 (produced in hybridomas; clone 9D9-mIgG2b), or negative controls (saline only and isotype control mAb). Injections were performed twice a week for 3 weeks (a total of 6 times). After 60 days of monitoring, (A) 7/10 mice were tumor-free in the Competitor X mAb-treated group, compared to (B) 9/9 mice tumor-free in the InvivoGen mAb-treated group. Additionally, no significant differences were observed in the health of the mice, with comparable body weight throughout the study (C). Most notably, after 6 injections, 30%* of the mice in the Competitor X mAb-treated group were euthanized due to excessive tumor volume (endpoint criteria), whereas complete tumor regression was observed in all mice in the InvivoGen mAb-treated group (D).
In summary, InvivoGen’s optimized Anti‑mCTLA4‑mIgG2a shows increased in vivo efficiency compared to Competitor X’s mAb, which features the commonly used IgG2b isotype, known to display low antitumor activity in vivo.
Back to the top