Agonistic and Antagonistic Effects of LPS on TLR4
Bacterial lipopolysaccharide (LPS) is the major structural component of the outer wall of all Gram-negative bacteria and a potent activator of the immune system.
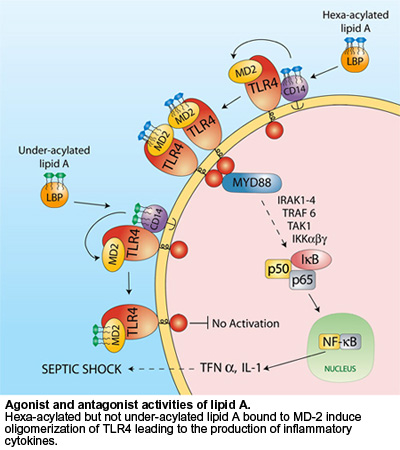
LPS can lead to pathological reactions such as the induction of septic shock. LPS is recognized by Toll-like receptor 4 (TLR4) which interacts with three different extracellular proteins: LPS binding protein (LBP), CD14 and, myeloid differentiation protein 2 (MD-2), to induce a signaling cascade leading to the activation of NF-κB and the production of proinflammatory cytokines.
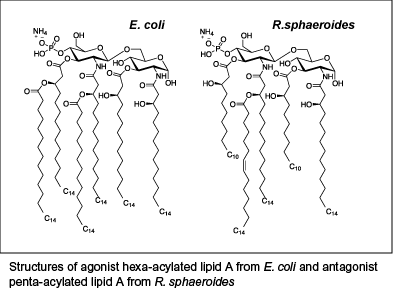
LPS structure
LPS consists of a polysaccharide region that is anchored in the outer bacterial membrane by a specific carbohydrate lipid moiety termed lipid A. Lipid A, also known as endotoxin, is responsible for the immunostimulatory activity of LPS. Lipid A is a glucosamine disaccharide linked to hydroxy fatty acids that are further substituted by nonhydroxylated fatty acids. The number of fatty acids is a major determinant of the immunogenicity of endotoxin. The most active form of lipid A contains six fatty acyl groups and is found in pathogenic bacteria such as Escherichia coli and Salmonella species. Underacylated lipid A structures, containing four or five fatty acids, induce markedly less host defense responses and can inhibit in a dose-dependent manner the strong endotoxic response triggered by hexa-acylated LPS. Such antagonist LPS have been isolated from Rhodobacter sphaeroides, Porphyromonas gingivalis and an E. coli strain bearing a mutation in the msbB gene[1].
LPS antagonists have received significant attention as potential therapeutic agents to treat septic shock long before their antagonist mechanism was known.
TLR4 activation pathway by LPS
According to the current model, LPS is delivered to CD14 by LBP and transferred to MD-2 to form a monomeric endotoxin:MD-2 complex that binds and activates TLR4 [2, 3]. TLR4 activation can occur without LPB and CD14 but requires several orders of magnitude more endotoxin. Canonical lipid A binds MD-2 and induces conformational changes that trigger TLR4 oligomerization and signaling.
Underacylated lipid A seem to utilize at least two distinct mechanisms to block LPS-dependent activation of TLR4. The main mechanism consists of direct competition between under-acylated LPS and hexa-acylated LPS for the same binding site on MD-2, while the secondary mechanism involves the ability of under-acylated LPS:MD-2 complexes to inhibit hexa-acylated endotoxin:MD-2 complexes function at TLR4 [1-4].
Understanding the molecular mechanism of LPS-induced TLR4 activation is key for the development of therapeutic lipid A antagonists. HEK293 cells transfected to stably express TLR4, MD-2 and CD14 is the model of choice to study TLR4 activation.
InvivoGen provides such cell line with or without a convenient reporter system to monitor TLR4-induced NF-κB activation, as well as a large collection of TLR4 agonists and antagonists.
References:
1. Coats SR. et al., 2005. MD-2 mediates the ability of tetra-acylated and penta-acylated lipopolysaccharides to antagonize Escherichia coli lipopolysaccharide at the TLR4 signaling complex. J Immunol.;175(7):4490-8.
2. Teghanemt A. et al., 2005. Molecular basis of reduced potency of underacylated endotoxins. J Immunol. 175(7):4669-76.
3. Visintin A. et al., 2005. Pharmacological inhibition of endotoxin responses is achieved by targeting the TLR4 coreceptor, MD-2. J Immunol. 175(10):6465-72.
4. Saitoh S. et al., 2004. Lipid Aantagonist, lipid IVa, is distinct from lipid A in interaction with Toll-like receptor 4 (TLR4)-MD-2 and ligand-induced TLR4 oligomerization. Int Immunol. 16(7):961-9.



