Human CTLA4 (CD152) Antibody - Ipilimumab Biosimilar
| Product | Unit size | Cat. code | Docs. | Qty. | Price | |
|---|---|---|---|---|---|---|
|
Anti-hCTLA4-hIgG1 Human CTLA4 (Ipilimumab) antibody - Human IgG1 |
Show product |
100 µg 10 x 100 µg |
hctla4-mab1
|
|
Anti-human CTLA4 - Ipilimumab biosimilar - CAS #477202-00-9
Anti-hCTLA4-hIgG1 is the biosimilar of the clinical antibody ipilimumab. Anti-hCTLA4-hIgG1 features the constant region of the human IgG1 isotype and the variable region of ipilimumab. Ipilimumab is a fully human IgG1 monoclonal antibody that targets CTLA-4 (also known as CD152), a negative regulator of T cell activation.
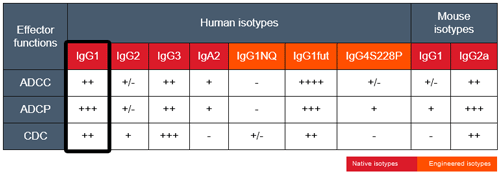
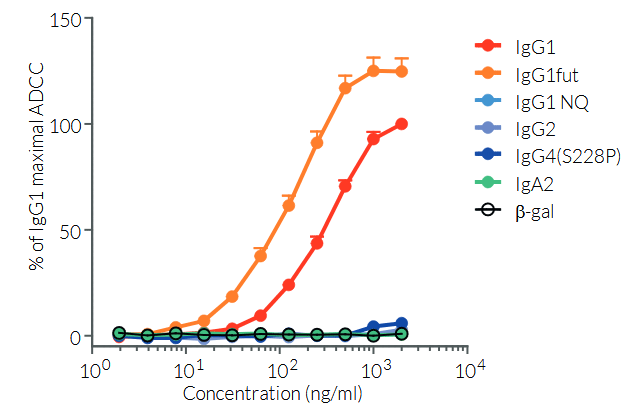
By binding CTLA-4, ipilimumab inhibits negative signals that physiologically downregulate T cell activation and exerts its therapeutic activity by upregulating the antitumor activity of T lymphocytes [1, 2]. In addition, Ipilimumab induces antibody-dependent cell-mediated cytotoxicity (ADCC) and TNF-α production [3]. Ipilimumab has been approved by the FDA for the treatment of unresectable or metastatic melanoma. Ipilimumab is undergoing clinical trials for other types of cancers, including lung cancer [4]. Human IgG1 is the most abundant immunoglobin present in serum and binds with high affinity to the Fc receptor on phagocytic cells. The human IgG1 isotype displays high ADCC and complement-dependent cytotoxicity (CDC).
Anti-hCTLA4-hIgG1 was generated by recombinant DNA technology. It has been produced in Chinese hamster ovary (CHO) cells and purified by affinity chromatography with protein G. More isotypes of this antibody are available and can be used for comparison of biological activities such as ADCC (see below or in the 'upon request' section).
References:
Grosso JF. & Jure-Kunkel MN., 2013. CTLA-4 blockade in tumor models: an overview of preclinical and translational research. Cancer Immun. 13:5.
Maio M. et al., 2013. Update on the role of ipilimumab in melanoma and first data on new combination therapies. Curr Opin Oncol. 25:166-72.
Laurent S.. et al., 2013. The engagement of CTLA-4 on primary melanoma cell lines induces antibody-dependent cellular cytotoxicity and TNF-α production. J Transl Med. 11:108.
Tomasini P., 2012. Ipilimumab: its potential in non-small cell lung cancer. Ther Adv Med Oncol. 4: 43–50.
Specifications
Application: Neutralization assay, flow cytometry, ELISA, Fc interaction studies
Isotype: Human IgG1, kappa
Recommended isotype control: Human IgG1
Target: Human CTLA-4 (CD152, CTLA4)
Species reactivity: Human
Clone: Ipilimumab, MDX-010, BMS-734016
Cas number: 477202-00-9
Sterility: 0.2 µm filtration
Source: CHO cells
Production: Animal-free
Purification: Protein A
Molecular weight: 145.4 kDa
Physical form: Lyophilized
Formulation buffer: Sodium phosphate buffer with glycine, saccharose, and stabilizing agents
Preservative: Azide-free
Reconstitution buffer: Sterile water (not provided)
Purity: ≥ 95 %
Quality control: Each lot is functionally tested and validated
Back to the topContents
Anti-hCTLA4-hIgG1 purified monoclonal antibody is provided azide-free and lyophilized. It is available in two quantities:
- hctla4-mab1: 100 µg
- hctla4-mab1-1: 10 x 100 µg (1 mg)
![]() The product is shipped at room temperature.
The product is shipped at room temperature.
![]() Upon receipt, store at -20°C.
Upon receipt, store at -20°C.
Tags: buy Ipilimumab (anti-CTLA-4) | Ipilimumab (anti-CTLA-4) supplier | purchase Ipilimumab (anti-CTLA-4) | Ipilimumab (anti-CTLA-4) cost | Ipilimumab (anti-CTLA-4) manufacturer | order Ipilimumab (anti-CTLA-4) | Ipilimumab (anti-CTLA-4) distributor
Back to the topDetails
The cytotoxic T-lymphocyte-associated protein 4 (CTLA-4, CD152) is an inhibitory receptor and immune checkpoint expressed by activated and regulatory T cells [1, 2].
The current paradigm is that full activation of T cells requires at least 2 signals upon contact with antigen-presenting cells (APCs) [3, 4]. Signal 1 is delivered upon the interaction of the T cell receptor (TCR) with antigenic peptides bound to major histocompatibility complex (MHC) molecules on antigen-presenting cells (APCs). Signal 2 is delivered upon the interaction of the co-stimulatory receptor CD28 with the B7 family ligands, B7-1 (CD80) and B7-2 (CD86), on APCs.
Signal 1: TCR and [HLA::peptide]
The 'classical' and most represented TCR is an 80 to 90 kDa heterodimer composed of one α chain and one β chain. The αβTCR is a transmembrane protein expressed by developing and mature T cells. It features an extracellular ligand-binding pocket and a short cytoplasmic tail. Each αβTCR is restricted to a specific complex made of an antigenic peptide and a class I or class II MHC molecule. Human MHC molecules are also known as HLA (human leukocyte antigen). Because of its short cytoplasmic tail, the TCR, once engaged, cannot signal and requires non-covalent association with the CD3 to trigger downstream intracellular signaling and T cell activation [3, 4]. Importantly, signal 1 without co-stimulation results in T cell unresponsiveness or 'anergy', a tolerance mechanism that guards against premature activation.
Signal 2: CD28 and CD80/86
CD28 is a homodimeric and transmembrane protein expressed by T cells. Nearly all human CD4+ T cells and 50% of human CD8+ T cells express CD28. The CD28 interaction with CD80 (aka B7-1) or CD86 (aka B7-2) on APCs, in conjunction with TCR engagement, triggers a co-stimulation signal (signal 2). It results in T-cell proliferation, cytokine production, cell survival, and cellular metabolism [3, 4].
IC signal: CTLA-4 and CD80/86
CTLA-4 exerts competitive binding to the co-stimulatory receptor CD28 ligands (i.e. CD80 and CD86) expressed by antigen-presenting cells. Thereby CTLA-4 upregulation by T cells prevents overstimulation.
Anti-CTLA-4 monoclonal antibodies (mAbs), as well as other immune checkpoints targeting mAbs, are extensively investigated to treat various cancers [2, 5, 6].
Ipilimumab background
Ipilimumab, a fully human IgG1 monoclonal antibody targets the Cytotoxic T-lymphocyte Associated Protein 4 (CTLA-4), also known as CTLA4 or CD152. CTLA-4 is an important immune checkpoint and negative regulator of T cell activation. Upon binding to CTLA-4, Ipilimumab inhibits negative signals that physiologically downregulate T cell activation and exerts its therapeutic activity by upregulating the antitumor activity of T lymphocytes [7,8]. In addition, Ipilimumab induces antibody-dependent cell-mediated cytotoxicity (ADCC) and TNF-α production [9]. Ipilimumab is FDA-approved for the treatment of unresectable or metastatic melanoma and is undergoing various clinical trials for other types of cancers, including lung cancer [10].
References:
1. Ribas A. and Wolchock J.D. 2018. Cancer immunotherapy using checkpoint blockade. Science. 359:1350.
2. Wei, S.C. et al. 2018. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 8(9):1069.
3. Budd R.C. & Fortner K.A., 2017. Chapter 12 - T Lymphocytes. Kelley and Firestein's Textbook of Rheumatology (Tenth Edition). pages 189-206.
4. Smith-Garvin J.E. et al., 2009. T Cell Activation. Ann. Rev. Immunol. 27:591-619.
5. Wilson, R.A.M. et al. 2018. Immune checkpoint inhibitors: new strategies to checkmate cancer. Clin. Exp. Immunol. 191(2):133-148.
6. Marin-Acevedo J.A. et al. 2018. Next generation of immune checkpoint therapy in cancer: new developments and challenges. J. Hematol. Oncol. 11(1):39.
7. Grosso JF. & Jure-Kunkel MN., 2013. CTLA-4 blockade in tumor models: an overview of preclinical and translational research. Cancer Immun. 13:5.
8. Maio M. et al., 2013. Update on the role of ipilimumab in melanoma and first data on new combination therapies. Curr Opin Oncol. 25:166-72.
9. Laurent S.. et al., 2013. The engagement of CTLA-4 on primary melanoma cell lines induces antibody-dependent cellular cytotoxicity and TNF-α production. J Transl Med. 11:108.
10. Tomasini P., 2012. Ipilimumab: its potential in non-small cell lung cancer. Ther Adv Med Oncol. 4: 43–50.