NATE™ - Transfection & Transduction Enhancer
| Product | Unit size | Cat. code | Docs. | Qty. | Price | |
|---|---|---|---|---|---|---|
|
NATE™ Nucleic Acid Transfection & Transduction Enhancer |
Show product |
1 mL (100 reactions) |
lyec-nate
|
|
Boost your transfection or transduction efficacy with NATE™
NATE™ - Nucleic Acid Transfection/Transduction Enhancer
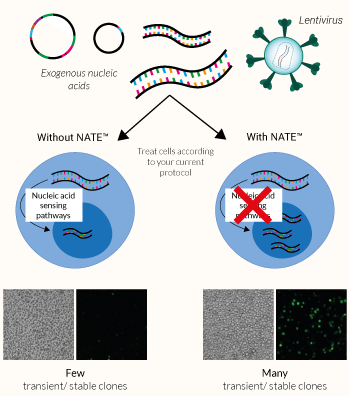
NATE™ is a non-toxic, highly efficient Nucleic Acid Transfection/Transduction Enhancer comprising a proprietary blend of innate immune system inhibitors. It has been designed by InvivoGen to boost transfection and/or transduction efficacy in:
– Immortalized cell lines (e.g. THP-1)
– Primary T cells
These cell types, especially immune cells, often express potent cytosolic sensors that detect foreign nucleic acids. The activation of these defensive signaling cascades frequently leads to low transfection/transduction yield, reduced cell viability, and inconsistent results. Therefore, an enhancer or booster is required to disable these defenses, helping facilitate the expression of nucleic acids and greatly improve gene transfer success.
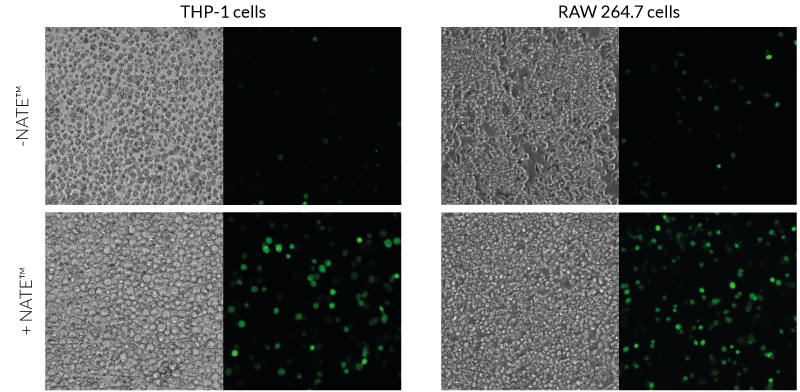
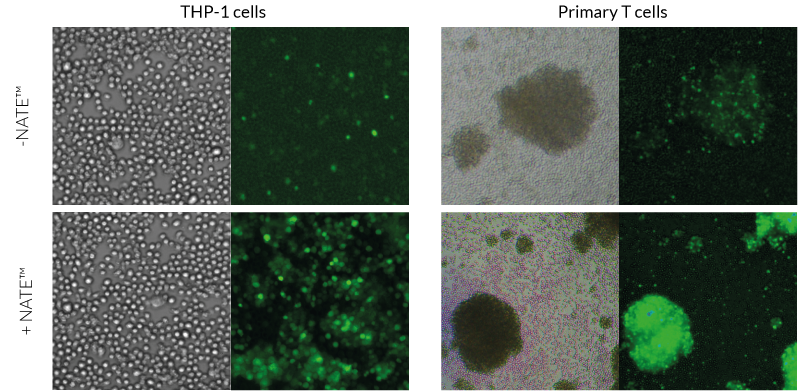
The NATE™ reagent blocks these restrictive nucleic acid sensing pathways, thereby protecting exogenous DNA and facilitating its expression. NATE™ is highly pure (>95%) and has been functionally tested in gene transfer assays using THP-1, RAW 264.7, and primary T cells (see figures).
Key features of NATE™ reagent
➔ Efficient
Improved gene transfer efficacy in hard-to-transfect cell lines (THP-1) and primary cells (T cells).
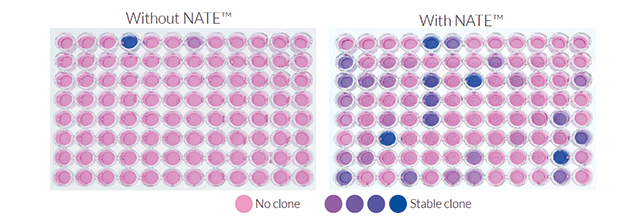
- Transfection: High yield even with large plasmids – stable or transient
- Transduction: Improved transduction and optimal copy number per cell with low MOI
➔ Easy to use
Simply add NATE™ to the cells 30 minutes before your commonly used protocol.
➔ Rapid
No extra pre-coating, pre-mixing step, or re-incubation cycles are necessary.
➔ Versatile
Compatible with common gene transfer methods (e.g. Lipofectamine® LTX, protamine sulfate, nucleofection, spinoculation, etc.).
➔ Cost-effective
1 ml of NATE™ is sufficient for 100 reactions (transduction or transfection).
➔ Gentle
No induced toxicity was observed in all tested protocols.
Background
The principal obstacle for ‘foreign’ nucleic acids (i.e. plasmids) during eukaryotic cell transfection is their detection by cytosolic DNA sensors of the innate immune system. These defensive cellular strategies can greatly affect the efficiency of both transient and stable transfections.
Back to the topSpecifications
Composition: Proprietary blend of innate immune system inhibitors
Purity: > 95% UHPLC
Quality Control:
- The absence of bacterial contamination (i.e. lipoproteins and endotoxin) has been confirmed using HEK-Blue™ TLR2 and TLR4 cellular assays, respectively.
- The biological activity of NATE™ has been confirmed using functional assays.
Back to the top
Contents
- 2 vials of NATE™ (approximately 100 reactions) provided in an evaporated form
![]() Product is shipped at room temperature
Product is shipped at room temperature
![]() Upon receipt product should be stored at -20°C
Upon receipt product should be stored at -20°C
Details
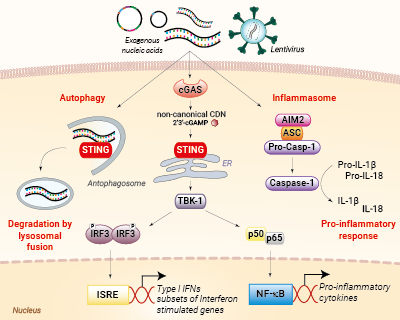
Nucleic acid sensing pathways encountered during gene transfer
The principal obstacle for ‘foreign’ nucleic acids (such as plasmid DNA) during eukaryotic cell transfection or viral transduction is their own detection by cytosolic nucleic acid sensors such as Absent in melanoma 2 (AIM2) and cyclic GMP-AMP synthase (cGAS) [1].
AIM2 is a cytosolic sensor that strongly responds to double-stranded DNA (dsDNA) leading to the formation of a protein complex called the inflammasome. Ultimately, the activation of the AIM2 inflammasome induces the maturation of the pro-inflammatory cytokines’ interleukin-1β (IL-1β) and IL-18 via caspase-1 cleavage. The production of these cytokines is detrimental to transfection success [1].
Furthermore, the sensing of dsDNA by cGAS induces the production of type I interferons (IFNs), through the STING/TBK1/IRF3 signaling axis, as well as the expression of several interferon-stimulated genes (ISGs), which exert potent effector functions, which are highly unwanted in transfection [1].
Additionally, the activation of STING can lead to LC3-dependent autophagy, through a TBK1- and IFN-independent mechanism [2]. Eventually, an autophagosome will form containing the 'foreign' nucleic acids (e.g. plasmid), and fuse with a lysosome for its clearance from the cell.
References:
1. Patrick, K.L. et al. 2016. For Better or Worse: Cytosolic DNA Sensing during Intracellular Bacterial Infection Induces Potent Innate Immune Responses. J Mol Biol 428, 3372-3386.
2. Gui, X. et al. 2019. Autophagy induction via STING trafficking is a primordial function of the cGAS pathway. Nature 567, 262-266.
Back to the top