WGP - Whole Glucan Particles
| Product | Unit size | Cat. code | Docs. | Qty. | Price | |
|---|---|---|---|---|---|---|
|
WGP Dispersible Dectin-1 Agonist |
Show product |
50 mg |
tlrl-wgp
|
|
||
|
WGP Soluble Dectin-1b Antagonist / WGP control |
Show product |
50 mg |
tlrl-wgps
|
|
Dectin-1 Agonist and Antagonist - WGP Dispersible and Soluble
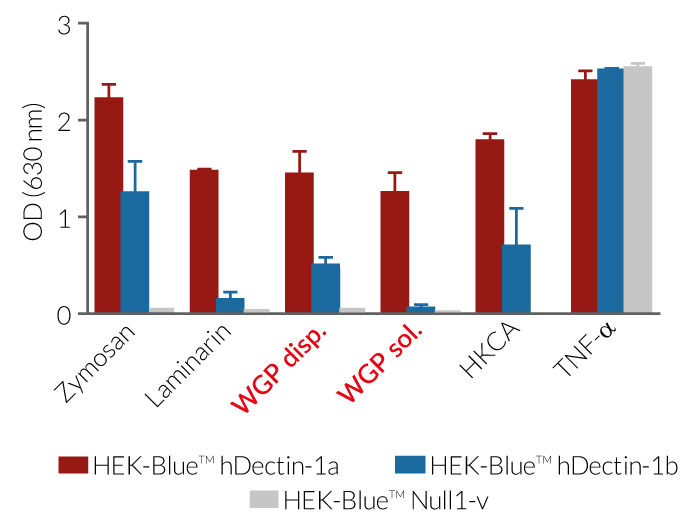
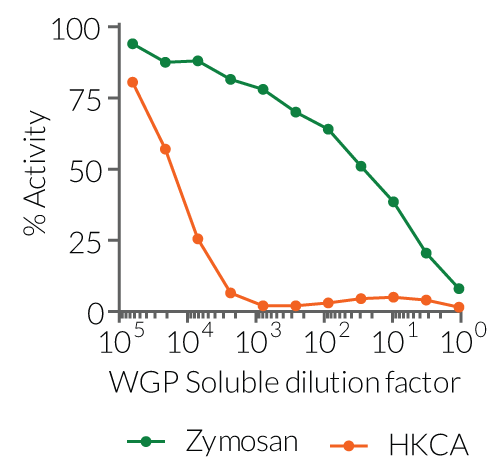
InvivoGen provides two preparations of whole glucan particles (WGP®, Biothera) for Dectin-1-receptor activation or inhibition:
— WGP Dispersible - particulate
— WGP Soluble (control) - soluble
These products are extracted from the cell wall of Saccharomyces cerevisiae after a series of alkaline and acidic extractions. They consist of hollow yeast cell wall “ghosts” composed mainly of long β-1,3 glucose polymers [1].
Both WGP preparations bind and activate the Dectin-1a receptor (long isoform) and are thus considered Dectin-1a-agonists (see figures). Additionally, the particulate WGP Dispersible binds and triggers Dectin-1b receptor (short isoform) signaling. The WGP Soluble can also bind to Dectin-1b. However, it is incapable of activating this receptor [1]. Furthermore, it is able to significantly block the binding of particulate WGP to macrophages and its immunostimulatory effect [2]. Therefore, it acts as an antagonist of Dectin-1b-induced response and can be used as a control for Dectin-1b-dependent signaling (see figures).
Dectin-1 is a C-type lectin receptor (CLR) that recognizes β-glucans and thus plays an important role in antifungal innate immunity [3]. β-Glucans, such as whole glucan particles (WGP), bind to Dectin-1a and Dectin-1b, inducing phagocytosis as well as the production of pro-inflammatory cytokines and reactive oxygen species (ROS). Unlike other yeast cell wall components such as Zymosan, WGP lack TLR-stimulating activity [2].
Key features:
- Potent agonist or antagonist of Dectin-1 signaling
- Can be used in murine or human CLR reporter cells
- Each lot is functionally tested
WGP® Dispersible and WGP® Soluble are registered trademarks of Biothera - Wellmune®.
![]() Read our review on Dectin-1 & β-glucans.
Read our review on Dectin-1 & β-glucans.
![]() Read our review on Dectin-1 & inflammasomes.
Read our review on Dectin-1 & inflammasomes.
References:
1. Li B. et al., 2007. Yeast glucan particles activate murine resident macrophages to secrete proinflammatory cytokines via MyD88- and Syk kinase-dependent pathways. Clin Immunol. 124(2):170-81.
2. Goodridge HS. et al., 2011. Activation of the innate immune receptor Dectin-1 upon formation of a 'phagocytic synapse'. Nature. 472(7344):471-5.
3. Heinsbroek S.E. et al., 2006. C-type Lectin Receptor: Old Friend and New Player. Med Chem. 2017;13(6):536-543.
Specifications
WGP Dispersible
Specificity: Dectin-1a and Dectin-1b agonist
Working concentrations: : 1 - 200 µg/ml
Quality Control:
- The biological activity has been validated using cellular assays.
- The absence of bacterial contamination (e.g. lipoproteins and endotoxins) has been confirmed using HEK-Blue™ TLR2 and HEK-Blue™ TLR4 cells.
WGP Soluble
Specificity: Dectin-1a agonist and Dectin-1b antagonist
Working concentrations: 1 - 1000 ng/ml
Quality Control:
- The biological activity has been validated using cellular assays.
- The absence of bacterial contamination (e.g. lipoproteins and endotoxins) has been confirmed using HEK-Blue™ TLR2 and HEK-Blue™ TLR4 cells.
Contents
Please note: each ligand is sold separately.
WGP Dispersible
- tlrl-wgp: 50 mg
WGP Soluble
- tlrl-wgps: 50 mg
![]() Products are shipped at room temperature.
Products are shipped at room temperature.
![]() Upon receipt, store at room temperature.
Upon receipt, store at room temperature.
Back to the top
Details
β-Glucans
β-Glucans are carbohydrates consisting of a backbone of glucose residues joined by β-(1➝3) linkages with β(1➝6) linked glucose side-chain residues. These polysaccharides are major cell wall structural components in fungi, including the yeasts Saccharomyces cerevisiae and Candida albicans. They are also found in plants and some bacteria. Depending on the source, β-glucans vary in the type of linkage, the degree of branching, molecular weight and tertiary structure. They are not synthesized by animals and thus are recognized by the innate immune system as pathogen-associated molecular patterns (PAMPs). This recognition is mediated by pattern recognition receptors (PRRs) and, among them, Dectin-1 has emerged as the primary receptor [1].
C-type lectin receptor Dectin-1
C-type lectin receptors (CLRs) comprise a large family of receptors that bind to carbohydrates in a calcium-dependent manner. CLRs include Dectin-1, Mincle, DC-SIGN, DC-SIGNR and MBL. These CLRs share one or more carbohydrate-recognition domains (CRD). They are involved in fungal recognition and the modulation of the innate immune response.
Dectin-1 is one member of the CLR family and plays an important role in antifungal innate immunity. It is expressed primarily by cells of myeloid origin, including macrophages, dendritic cells and neutrophils. In human and mice, Dectin-1 is alternatively spliced into 2 major isoforms: a full-length A isoform (Dectin-1a) and a ‘stalkless’ B isoform (Dectin-1b). Dectin-1b does not induce the same response to soluble and particulate β-glucans [2].
Upon binding to its ligand, Dectin-1 triggers phagocytosis and activation of Src and Syk kinases, through its ITAM-like motif. Syk, in turn, induces the CARD9-Bcl10-Malt1 complex leading to the production of reactive oxygen species (ROS), activation of NFκB and subsequent secretion of proinflammatory cytokines [3]. ROS have a direct microbicidal role in the phagosome but also can affect IL-1β secretion by activating the NLRP3 inflammasome, which in turn activates caspase-1 and permits processing of pro-IL-1β [4]. Dectin-1 signaling has been shown to collaborate with Toll-like receptor 2 (TLR2) signaling to enhance the responses triggered by each receptor [5].
Indeed, most published studies focus exclusively on the responses of murine (m)Dectin-1 to β-glucans, which may differ considerably from the response mediated by human (h)Dectin-1. In addition to discrepancies between mDectin-1 and hDectin-1, many inconsistencies exist due to the use of very different, and often impure β-glucans, and the analysis of different cell types and model systems. Recognition of β-glucans by the immune system appears very complex and further studies are required to fully understand the immunomodulating properties of these molecules.
References:
1. Tsoni SV & Brown GD. 2008. β-Glucans and dectin-1. Ann N Y Acad Sci. 1143:45-60.
2. Heinsbroek S.E. et al., 2006. C-type Lectin Receptor: Old Friend and New Player. Med Chem. 2017;13(6):536-543.
3. Goodridge HS. et al., 2011. Activation of the innate immune receptor Dectin-1 upon formation of a 'phagocytic synapse'. Nature. 472(7344):471-5.
4. Kankkunen P. et al., 2010. (1,3)-b-glucans activate both Dectin-1 and NLRP3 inflammasome in human macrophages. J Immunol. 184;6335-6342.
5. Gantner BN. et al., 2003. Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J Exp Med. 197(9):1107-17.