Oxidative Stress ARE Lucia Reporter Cells (Nrf2 KEAP1 Pathway)
| Product | Unit size | Cat. code | Docs. | Qty. | Price | |
|---|---|---|---|---|---|---|
|
HEK-Lucia-Star™ ARE Cells Oxidative stress HEK293 reporter cell line (Nrf2 KEAP1 pathway) |
Show product |
3-7 x 10e6 cells |
hkls-are
|
|
ROS Reporter Cells - Nrf2-KEAP1 pathway
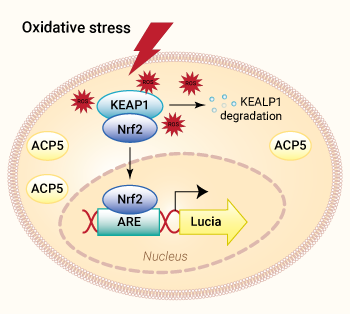
HEK-Lucia-Star™ ARE cells signaling pathway
HEK-Lucia-Star™ ARE cells were engineered from the human embryonic kidney HEK 293 cell line. They were designed to monitor the activation of the Nrf2-ARE (antioxidant-responsive element) pathway in response to oxidative stress.
Oxidative stress results from an imbalance between the production of reactive oxygen species (ROS) and the ability of cells to scavenge them. High levels of ROS can lead to an apoptosis-like cell death phenomenon called oxeiptosis. ROS play an important role in the progression of several diseases, including diabetes, atherosclerosis, aging, and cancer [1-2].
Cell line description
The HEK-Lucia-Star™ ARE cell line features the inducible Lucia® luciferase gene under the control of a minimal promoter fused to multimeric antioxidant response elements (ARE). Upon oxidative stress induction, Lucia® luciferase levels can be monitored in the supernatant.
Moreover, these cells constitutively express a secreted version of the tartrate-resistant acid phosphatase 5 (TRAP, ACP5). Measuring the ACP5 level in the supernatant while performing compound screening allows you to assess cell viability simultaneously.
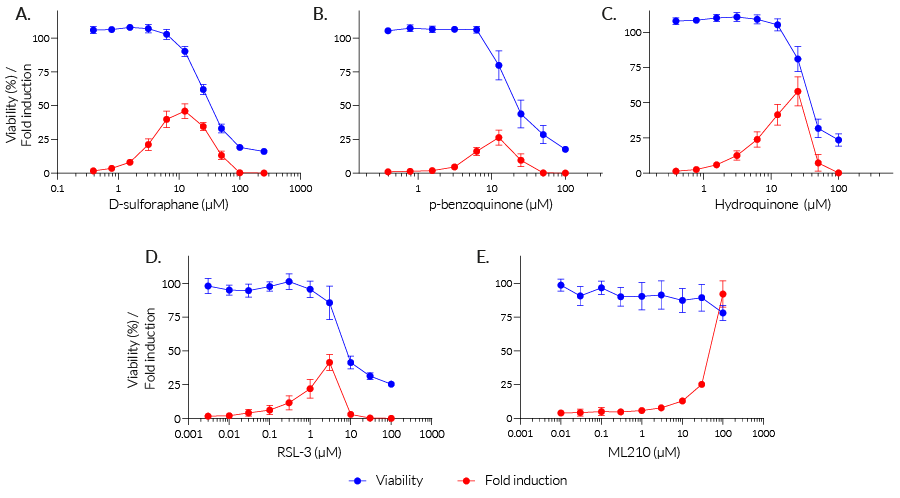
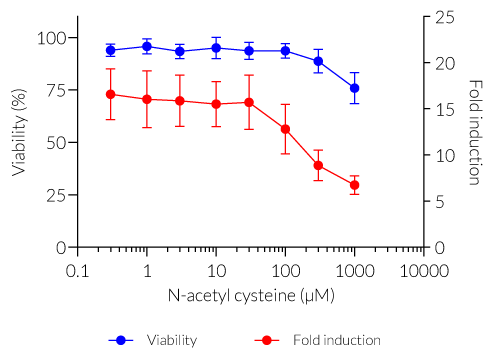
Stimulation with increasing concentrations of oxidant compounds (D-sulforaphane, p-benzoquinone, hydroquinone) or peroxidase inhibitors (RSL-3, ML210) strongly induces oxidative stress responses in HEK-Lucia-Star™ ARE cells (see figure, fold response in red). The loss of the secreted ACP5 signal at higher concentrations of these compounds indicates oxidative stress-induced cell death (see figure, viability percentage in blue). Interestingly no cell death was observed at even the highest concentration of ML210. Treatment with the antioxidant compound N-acetylcysteine decreased the oxidative stress response in HEK-Lucia-Star™ ARE cells in a dose-dependent manner (see figure).
For your convenience, InvivoGen provides easy-to-use liquid detection reagents, QUANTI-Luc™ 4 for Lucia® and QUANTI-Star™ for ACP5, to evaluate the modulatory impact of your compound. Thus, assessing the Lucia® and ACP5 activities provides direct information about the oxidative properties and toxicity effects of your compound of interest.
Features
- Stable expression of two secreted reporter proteins, Lucia® luciferase and ACP5
- Fully functional ARE-dependent Nrf2 KEAP1 pathway
- Readily assessable oxidative stress-inducible Lucia® reporter activity
- Concomitant surveillance of cell viability via ACP5 reporter activity
- Reporter activities measurable in the supernatant (no lysis needed)
Applications
- Studying oxeiptosis and the ARE-dependent pathway via Nrf2 and KEAP1
- Screening of novel ligands with oxidative or antioxidant properties
- Simultaneous monitoring of compound-induced cell death via ACP5 activity
Reference:
1. Hecht F, et al., 2024. Regulation of antioxidants in cancer. Mol Cell.;84(1):23-33.
2. Pichlmair A, et al., 2018. Oxeiptosis, a ROS-induced caspase-independent apoptosis-like cell-death pathway. Nat Immunol.(2):130-140.
Specifications
Antibiotic resistance: Hygromycin and Zeocin®
Growth medium: DMEM, 4.5 g/l glucose, 2-4 mM L-glutamine, 10% (v/v) fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin, 100 μg/ml Normocin™
Quality Control:
- The Lucia luciferase and ACP5 reporter activity were validated in response to various ligands.
- The stability for 20 passages, following thawing, is verified.
- These cells are guaranteed mycoplasma-free.
Contents
- 1 vial containing 3-7 x 106 cells
- 1 ml Hygromycin B Gold (100 mg/ml)
- 1 ml Normocin™ (50 mg/ml)
- 1 ml Zeocin® (100 mg/ml)
- 1 tube of QUANTI-Luc™ 4 Reagent, a Lucia luciferase detection reagent (sufficient to prepare 25 ml)
- 0.5 ml of QUANTI-Star™ reagent and 0.5 ml of QUANTI-Star™ buffer (sufficient to prepare 50 ml of QUANTI‑Star™ Solution, a ACP5 detection reagent)
![]() Shipped on dry ice (Europe, USA, Canada, and some areas in Asia)
Shipped on dry ice (Europe, USA, Canada, and some areas in Asia)
FAQ Cell Lines
 Any questions about our cell lines ? Visit our frequently asked questions page
Any questions about our cell lines ? Visit our frequently asked questions page
Back to the top
Details
Introduction
Oxidative stress refers to the imbalance between the production of reactive oxygen species (ROS) and the ability of the body to counteract their harmful effects through neutralization by antioxidants [1]. ROS, including free radicals such as superoxide anions, hydrogen peroxide, and hydroxyl radicals, are byproducts of normal cellular metabolism, particularly from mitochondrial oxidative phosphorylation [2]. While ROS play essential roles in cell signaling and homeostasis, excessive amounts can damage cellular components such as lipids, proteins, and DNA, finally leading to oxeiptosis, an apoptosis-like cell death [3]. They are also involved in various pathologies including cancer, neurodegenerative diseases, cardiovascular diseases, and aging [4].
Nrf2-Keap1 pathway
The nuclear factor erythroid 2–related factor 2 (Nrf2) is a critical transcription factor that regulates the expression of antioxidant proteins that protect against oxidative damage triggered by injury and inflammation. Nrf2 controls the expression of a set of genes involved in the antioxidant response, detoxification processes, and maintenance of cellular redox homeostasis [2].
Kelch-like ECH-associated protein 1 (KEAP1) is an E3 ligase adaptor protein and the gatekeeper of Nrf2. It serves as a negative regulator of Nrf2. Under normal conditions, Keap1 binds to Nrf2 in the cytoplasm, promoting its ubiquitination and subsequent proteasomal degradation. This tight regulation ensures that Nrf2 levels remain low under homeostatic conditions, preventing unwarranted antioxidant response [2].
Under moderate oxidative or electrophilic stress, the cysteine residues of KEAP1 undergo modification, leading to a conformational change that impairs its ability to target Nrf2 for degradation. This allows Nrf2 to accumulate in the cytoplasm, translocate into the nucleus, and bind to the antioxidant response element (ARE) in the promoter regions of target genes, such as antioxidant enzymes, detoxification enzymes, glutathione, and proteasomal proteins [2].
Oxeiptosis
Interestingly, KEAP1 was found to be a concentration-dependent ROS sensor, that - when the oxidative stress is too high - can mediate an apoptosis-like, caspase-independent cell death called oxeiptosis [3]. This process is Nrf2-independent and characterized by activating KEAP1, phosphoglycerate mutase 5 (PGAM5), and apoptosis-inducing factor mitochondria-associated 1 (AIFM1) - short the KEAP1/PGAM5/AIFM1 pathway [3]. When ROS concentrations are elevated, PGAM5 dissociates from KEAP1 and migrates to the mitochondria. Inside the mitochondria, PGAM5 dephosphorylates the Ser116 residues of AIFM1, finally leading to oxeiptosis; Signaling downstream of AIFM1 which results in cell death is still enigmatic and remains to be elucidated [3].
Nrf2-Keap1 pathway in disease and therapeutics
Due to its crucial role in cellular anti-oxidant defense, the Nrf2-KEAP1 pathway became an attractive therapeutic target in a plethora of diseases [2-5].
In neurodegenerative diseases such as Alzheimer's and Parkinson's, oxidative stress plays a pivotal role in disease progression. Enhancing Nrf2 activity has shown potential in reducing oxidative damage and improving neuronal survival [4].
The role of Nrf2 in cancer is complex and double-edged. While Nrf2 activation protects normal cells from oxidative damage and reduces cancer risk, in established cancers, Nrf2 can promote tumorigenesis and chemoresistance by creating a reductive environment favorable for cancer cell proliferation. Furthermore, the activation of Nrf2 has been proposed as a host-directed therapeutic strategy to treat infectious diseases including COVID-19 [5].
Several therapeutics that aim to modulate the NRF2-KEAP1 pathway are under investigation [4-5]. The first US FDA-approved Nrf2 activator, DMF, has been successfully marketed as a multiple sclerosis (MS) treatment with impressive performance [5]. The modulation of this pathway offers a promising strategy for enhancing cellular defense mechanisms and reducing oxidative stress associated with a wide range of pathological conditions.
References:
1. Hecht F, et al., 2024. Regulation of antioxidants in cancer. Mol Cell.;84(1):23-33.
2. Song MY, et al., 2021. The Role of NRF2/KEAP1 Signaling Pathway in Cancer Metabolism. Int J Mol Sci.22(9):4376.
3. Pichlmair A, et al., 2018. Oxeiptosis, a ROS-induced caspase-independent apoptosis-like cell-death pathway. Nat Immunol.(2):130-140.
4. Cuadrado A, et al., 2019. Therapeutic targeting of the NRF2 and KEAP1 partnership in chronic diseases. Nat Rev Drug Discov. (4):295-317.
5. Wang J, et al., 2021. The potential roles of Nrf2/Keap1 signaling in anticancer drug interactions. Curr Res Pharmacol Drug Discov. 2:100028.