CR3018-derived Anti SARS-CoV-2 Nucleocapsid hIgG1 antibody
| Product | Unit size | Cat. code | Docs. | Qty. | Price | |
|---|---|---|---|---|---|---|
|
Anti-CoV-N-hIgG1 SARS-CoV nucleocapsid monoclonal human IgG1 antibody (Clone CR3018) |
Show product |
3 x 100 µg |
covn-mab1-3
|
|
SARS-CoV-2 nucleocapsid recombinant antibody
Antibody Description
Anti-CoV-N-hIgG1 is a SARS-CoV-2 recombinant monoclonal antibody (mAb) originally described as 'clone CR3018' [1]. It features:
- The variable region from 'clone CR3018' that specifically targets the nucleocapsid of SARS-CoV-2 [1]
- The human IgG1 constant region
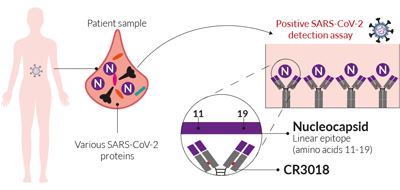
SARS-CoV-2 nucleocapsid detection mAb (clone CR3018)
The nucleocapsid (N) is an intraviral protein that plays a crucial role in viral infection through its involvement in RNA packaging and virus particle release [2]. It has been demonstrated that COVID-19 patients’ sera contain high levels of antibodies against the N protein, second only to the antigenic Spike protein [3]. Furthermore, it has been established that the N protein is highly expressed during SARS-CoV-2 infection, and is also a particularly sensitive marker for early diagnosis. [4].
During the previous SARS-CoV pandemic, a mAb targeting the nucleocapsid protein, clone CR3018, was isolated from semi-synthetic antibody phage display libraries [1]. Subsequently, CR3018 was shown to effectively bind to the SARS-CoV nucleocapsid protein in vitro. Furthermore, CR3018 was described to specifically bind with a linear epitope (amino acids 11-19) [1]. Importantly, this linear epitope is conserved in SARS‑CoV-2. Therefore, CR3018 binds to and recognizes the SARS‑CoV-2 nucleocapsid protein, and could be a useful tool for early detection.
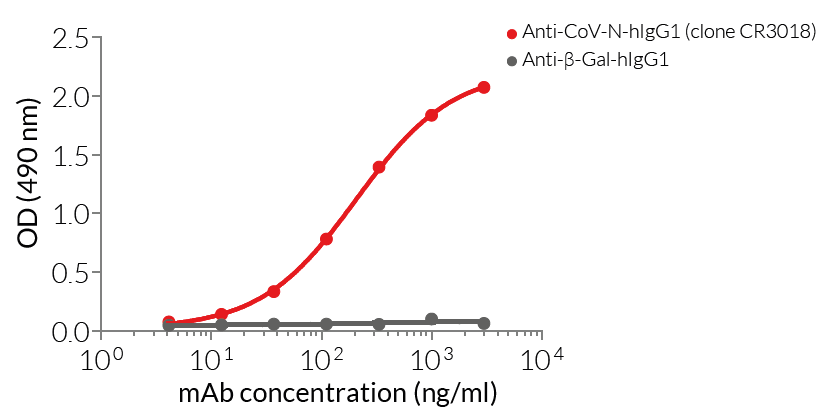
Anti-CoV-N-hIgG1 mAb has been generated by recombinant DNA technology, produced in CHO cells, and purified by affinity chromatography, ensuring lot-to-lot reproducibility. Furthermore, in house data has validated the binding of Anti-CoV-N-hIgG1 to the purified SARS-CoV-2 nucleocapsid protein (see figures). Cellular assays have confirmed the absence of bacterial contamination.
Applications
- Detecting the presence of SARS-CoV/ SARS-CoV-2 nucleocapsid protein in culture supernatant and/or in serum (ELISA)
Quality control
- Functionally validated by ELISA using a coated Nucleocapsid-His fusion protein
References
1. van den Brink, E.N. et al. 2005. Molecular and biological characterization of human monoclonal antibodies binding to the spike and nucleocapsid proteins of severe acute respiratory syndrome coronavirus. J Virol 79, 1635-1644.
2. Zeng, W. et al. 2020. Biochemical characterization of SARS-CoV-2 nucleocapsid protein. Biochem Biophys Res Commun 527, 618-623.
3. Burbelo, P.D. et al. 2020. Sensitivity in the Detection of Antibodies to Nucleocapsid and Spike Proteins of SARS-CoV-2 in Patients With COVID-19. J Infect Dis 222, 206-213.
4. Li, T. et al. 2020. Serum SARS-COV-2 Nucleocapsid Protein: A Sensitivity and Specificity Early Diagnostic Marker for SARS-COV-2 Infection. Front Cell Infect Microbiol 10, 470.
Specifications
Specificity: SARS-CoV & SARS-CoV-2 nucleocapsid
Clonality: Monoclonal
Source: CHO cells
Formulation: 0.2 μm filtered solution in a sodium phosphate buffer with glycine, saccharose, and stabilizing agents.
Purification: Affinity chromatography with protein G
Quality control:
- The complete sequence of the antibody construct has been verified.
- Antibody binding has been validated by ELISA using a coated SARS-CoV-2 Nucleocapsid-His fusion protein.
- The absence of bacterial contamination (e.g. lipoproteins and endotoxins) has been confirmed using HEK-Blue™ TLR2 and HEK-Blue™ TLR4 cellular assays.
Contents
- 3 x 100 µg purified antibody, azide-free, and lyophilized
![]() The product is shipped at room temperature.
The product is shipped at room temperature.
![]() Upon receipt, store lyophilized antibody at -20 °C.
Upon receipt, store lyophilized antibody at -20 °C.
Back to the top