LY-CoV2-derived Anti-SARS-CoV-2 RBD monoclonal antibodies
| Product | Unit size | Cat. code | Docs. | Qty. | Price | |
|---|---|---|---|---|---|---|
|
Anti-CoV2RBD-bam-hIgG1 Recombinant monoclonal hIgG1 (Bamlanivimab-derived) |
Show product |
3 x 100 µg |
srbdc5-mab1-3
|
|
||
|
Anti-CoV2RBD-bam-mIgG2a Recombinant monoclonal mouse IgG2a (Bamlanivimab-derived) |
Show product |
3 x 100 µg |
srbdc5-mab10-3
|
|
||
|
Anti-CoV2RBD-ete-hIgG1 Recombinant monoclonal human IgG1 (Etesevimab-derived) |
Show product |
3 x 100 µg |
srbdc6-mab1-3
|
|
||
|
Anti-CoV2RBD-ete-mIgG2a Recombinant monoclonal mouse IgG2a (Etesevimab-derived) |
Show product |
3 x 100 µg |
srbdc6-mab10-3
|
|
Specific SARS-CoV-2 Spike-RBD recombinant human IgG1 & mouse IgG2a antibodies
SARS-CoV-2 specific neutralization by Bamlinivimab & Etesevimab
Antibody description
InvivoGen provides a set of recombinant monoclonal antibodies (mAbs) featuring either a human IgG1 or a mouse IgG2a constant region, and the variable region of 'Bamlanivimab (LY-CoV555)' or 'Etesevimab (LY-CoV016'), two clones originally described as potent SARS-CoV-2 neutralizing mAbs [1,2]:
— Anti-CoV2RBD-bam-hIgG1 and Anti-CoV2RBD-bam-mIgG2a (derived from Bamlavinimab)
— Anti-CoV2RBD-ete-hIgG1 and Anti-CoV2RBD-ete-mIgG2a (derived from Etesevimab)
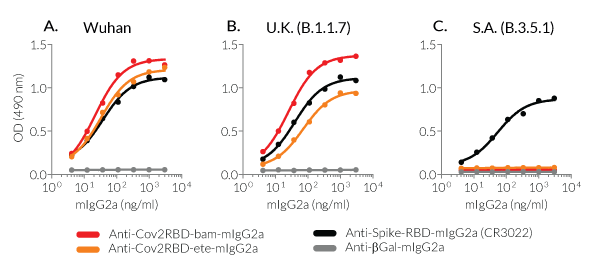
InvivoGen's Anti-CoV2RBD-bam and Anti-CoV2RBD-ete mAbs have been generated by recombinant DNA technology, produced in CHO cells, and purified by affinity chromatography, ensuring lot-to-lot reproducibility. Furthermore, these mAbs have been functionally validated by ELISA (see data below). The absence of bacterial contamination has been confirmed by cellular assays.
➔ These antibodies have been functionally validated by ELISA and FACS on SARS-CoV2 Spike variants.
Background
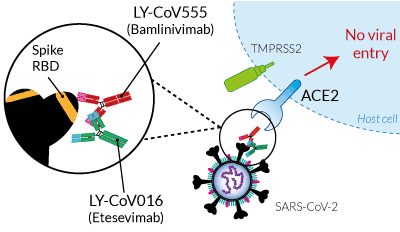
Bamlavinimab and Etesevimab (originally named 'clone CB6') were generated using serum from a convalescent patient [1, 2]. These mAbs specifically target two distinct but overlapping regions of the SARS-CoV-2 S-RBD and potently block its binding to the ACE2 receptor on target cells [1, 2].
![]() More details on Bamlavinimab and Etesevimab
More details on Bamlavinimab and Etesevimab
Applications
- Detecting the presence of SARS-CoV-2 in culture supernatant and/or in serum (ELISA)
- Flow cytometry binding assays
- Developing neutralizing antibody cocktails against SARS-CoV-2
- Monitoring SARS-CoV-2 variant escape
Quality control
- Functionally validated by ELISA using Spike-RBD proteins derived from SARS-CoV-2 variants and either HRP or Luciferase detection
References
1. Jones B.E. et al., 2021. The neutralizing antibody, LY-CoV555, protects against SARS-CoV-2 infection in nonhuman primates. Sci Transl Med 13(593), eabf1906(2021).
2. Shi R. et al., 2020. A human neutralizing antibody targets the receptor-binding site of SARS-CoV-2. Nature. 584:120-124.
Specifications
Specificity: SARS-CoV-2 Spike RBD
Clonality: Monoclonal
Isotypes: hIgG1 or mIgG2a
Source: CHO cells
Formulation: 0.2 μm filtered solution in a sodium phosphate buffer with glycine, saccharose, and stabilizing agents.
Purification: purified by affinity chromatography with protein G (hIgG1) or protein A (mIgG2a)
Quality control:
- The complete sequence of each antibody construct has been verified.
- Antibody binding has been validated by ELISA using Spike-RBD proteins derived from SARS-CoV-2 variants and either HRP or Luciferase detection.
- The absence of bacterial contamination (e.g. lipoproteins and endotoxins) has been confirmed using HEK-Blue™ TLR2 and HEK-Blue™ TLR4 cellular assays.
Contents
Note: Each antibody is sold separately.
- 3 x 100 µg purified antibody, azide-free, and lyophilized
![]() The product is shipped at room temperature.
The product is shipped at room temperature.
![]() Upon receipt, store at -20 °C.
Upon receipt, store at -20 °C.
Details
Bamlavinimab and Etesevimab (originally named 'clone CB6') were generated using serum from a convalescent patient [1, 2]. These mAbs specifically target two distinct but overlapping regions of the SARS-CoV-2 S-RBD and potently block its binding to the ACE2 receptor on target cells [1, 2]. Prophylactic administration of Bamlavinimab protects against SARS-CoV-2 infection in non-human primates [1]. Treatment using Bamlavinimab has been authorized under emergency use for COVID-19 outpatients [3, 4]. In addition, a clinical trial (NCT04427501) is investigating the efficacy of Bamlavinimab and Etesevimab combination.
Learn more about the SARS-CoV-2 Spike protein.
References
1. Jones B.E. et al., 2021. The neutralizing antibody, LY-CoV555, protects against SARS-CoV-2 infection in nonhuman primates. Sci Transl Med 13(593), eabf1906(2021).
2. Shi R. et al., 2020. A human neutralizing antibody targets the receptor-binding site of SARS-CoV-2. Nature. 584:120-124.
3. Chen P. et al., 2020. SARS-CoV-2 Neutralizing Antibody LY-CoV555 in Outpatients with Covid-19. N Engl J Med. 384(3):229-237.
4. https://www.fda.gov/media/145610/download.