CR3022-derived Anti-SARS-CoV2 RBD mAbs, human & mouse isotypes
| Product | Unit size | Cat. code | Docs. | Qty. | Price | |
|---|---|---|---|---|---|---|
|
Anti-Spike-RBD-hIgG1 (CR3022) Recombinant monoclonal human IgG1 antibody against Spike RBD |
Show product |
3 x 100 µg |
srbd-mab1-3
|
|
||
|
Anti-Spike-RBD-hIgG1NQ (CR3022) Recombinant monoclonal human IgG1NQ antibody against Spike RBD, non-glycosylated |
Show product |
3 x 100 µg |
srbd-mab12-3
|
|
||
|
Anti-Spike-RBD-hIgM (CR3022) Recombinant monoclonal human IgM antibody against Spike RBD |
Show product |
3 x 100 µg |
srbd-mab5-3
|
|
||
|
Anti-Spike-RBD-hIgA1 (CR3022) Recombinant monoclonal human IgA1 antibody against Spike RBD |
Show product |
3 x 100 µg |
srbd-mab6-3
|
|
||
|
Anti-Spike-RBD-mIgG2a (CR3022) Recombinant monoclonal mouse IgG2a antibody against Spike RBD |
Show product |
3 x 100 µg |
srbd-mab10-3
|
|
||
|
Anti-Spike-RBD-mIgG1e3 (CR3022) Recombinant monoclonal mouse IgG1e3 antibody against Spike RBD |
Show product |
3 x 100 µg |
srbd-mab15-3
|
|
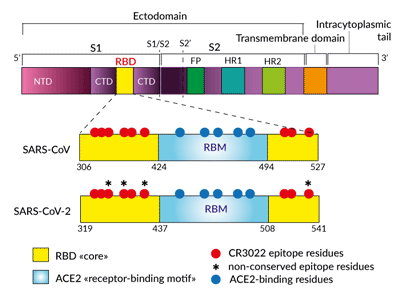
Simplified schematic of CR3022- and
ACE2-binding sites in Spike RBD
 Available upon request:
Available upon request:
• Anti-Spike-RBD-hIgG1-HRP (CR3022)
HRP-labelled Anti-Spike mAb -  TDS
TDS
Anti-SARS-CoV-2 & SARS-CoV Spike recombinant antibody
Antibody description
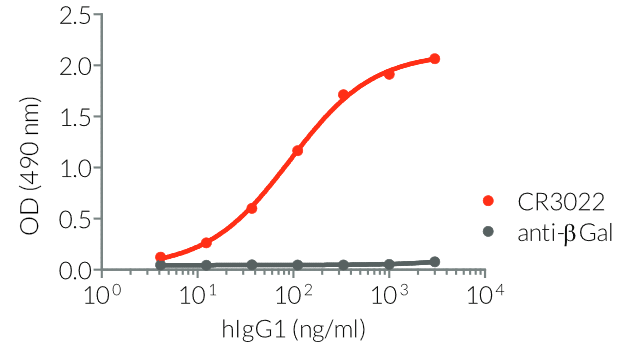
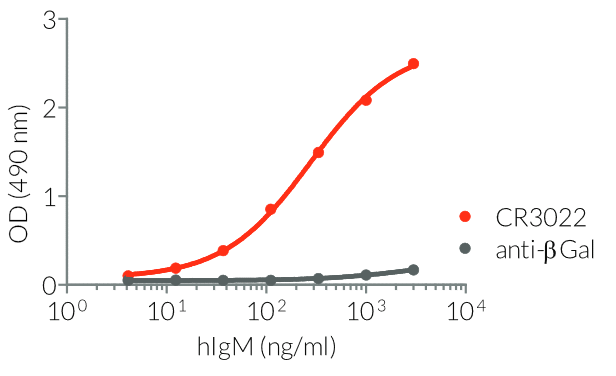
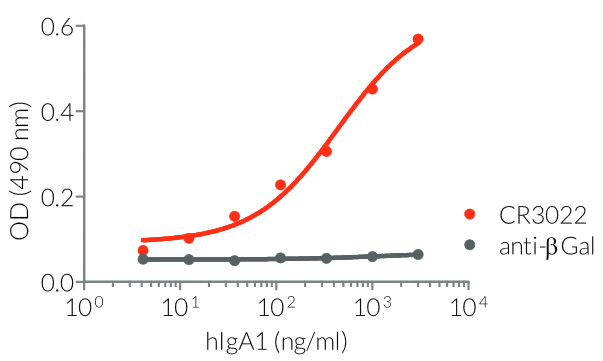
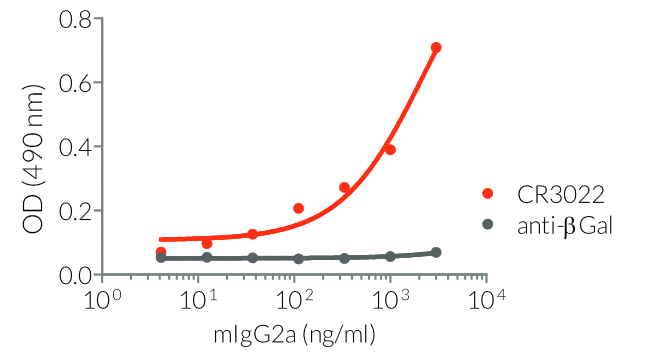
InvivoGen provides a family of Anti-Spike RBD CR3022-derived monoclonal antibodies (mAbs) in multiple human and mouse isotypes. These mAbs feature:
- the variable region of the CR3022 human mAb that specifically targets the Spike receptor-binding domain (RBD) [1]
- a constant region that mediates different effector functions depending on the isotype (human IgG1, hIgG1NQ, hIgA1, or hIgM, and mouse IgG2a or mIgG1e3 ) (See Table)
The Anti-Spike RBD clone CR3022 is a SARS-CoV neutralizing antibody that was obtained from the screening of an antibody-phage library and converted to a human IgG1 format [1]. SARS-CoV and SARS-CoV-2 (2019-nCoV) RBD share a highly conserved epitope (86% shared identity) that is recognized by CR3022 [2, 3]. To date, the neutralization potency of CR3022 for SARS-CoV-2 is still unclear [3, 4]. Indeed, CR3022 does not interfere with the ACE2 binding motif [2-4].
Applications
- Detection of the presence of viral particles in culture supernatant or in serum (ELISA)
- Control of antibody-mediated effector functions (i.e. absence, increase or decrease in ADCC, ADCP, or CDC) (See Table)
Quality control
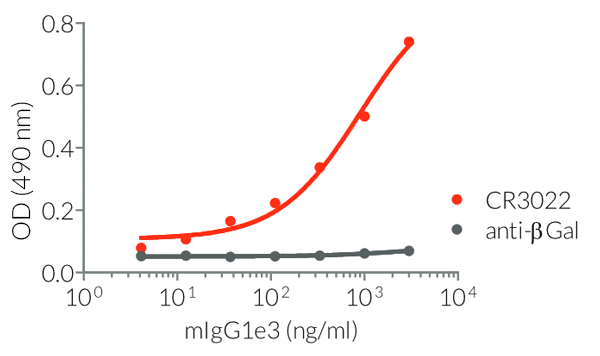
- Isotype confirmed by ELISA
- Functionality validated by ELISA using a coated Spike-RBD-His fusion peptide
Our CR3022 variants have been generated by recombinant DNA technology, produced in CHO cells, and purified by affinity chromatography.
All the SARS-CoV-2-related antibodies are in stock now and can be shipped out immediately.
![]() Read our reviews discussing SARS-CoV-2
Read our reviews discussing SARS-CoV-2
References
1. Ter Muelen J. et al., 2006. Human monoclonal antibody combination against SARS coronavirus: synergy and coverage of escape mutants. PLos Med. 3(7):e237.
2. Yuan M. et al., 2020. A highly conserved cryptic epitope in the receptor-binding domains of SARS-CoV-2 and SARS-CoV. Science. DOI: 10.1126/science.abb7269.
3. Tian X. et al., 2020. Potent binding of 2019 novel coronavirus spike protein by a SARS coronavirus-specific human monoclonal antibody. Emerging Microbes & Infections. 9(1):382-385.
4. Huo J. et al., 2020. Neutralization of SARS-CoV-2 by destruction of the prefusion Spike. bioRxiv. DOI:10.1101/2020.05.05.079202.
Specifications
Specificity: Target SARS-CoV and SARS-CoV-2 Spike RBD
Clonality: Monoclonal antibodies
Source: CHO cells
Formulation: 0.2 μm filtered solution in a sodium phosphate buffer with glycine, saccharose, and stabilizing agents.
Purification:
- hIgG1 and hIgG1NQ: purified by affinity chromatography with protein G
- hIgA1: purified by affinity chromatography with peptide M
- hIgM: purified by affinity chromatography with protein L
- mIgG1e3 and mIgG2a: purified by affinity chromatography with protein A
Quality control:
- The complete sequence of each antibody construct has been verified.
- Each antibody isotype has been confirmed by ELISA.
- The functionality of each antibody has been validated by ELISA using a coated Spike-RBD-His fusion peptide.
- The absence of bacterial contamination (e.g. lipoproteins and endotoxins) has been confirmed using HEK-Blue™ TLR2 and HEK-Blue™ TLR4 cellular assays.
Contents
- 3 x 100 µg purified antibody, azide-free, and lyophilized
![]() The product is shipped at room temperature.
The product is shipped at room temperature.
![]() Store lyophilized antibody at -20°C.
Store lyophilized antibody at -20°C.
Details
The anti-Spike RBD clone CR3022 is a SARS-CoV neutralizing antibody that was obtained from the screening of an antibody-phage library and converted to a human IgG1 format [2]. SARS-CoV-2 and SARS-CoV Spike RBD share 73% identity [3]. Modeling and in vitro studies have shown that CR3022 is also binding to SARS-CoV-2 RBD [4]. This cross-reactivity is explained by the 86% shared amino acid identity between the CR3022 epitopes from the two viruses [4]. Importantly, CR3022 does not interfere with the ACE2 binding motif [2-5]. Thus, CR3022 could be used alone or in combination with other antibodies or soluble ACE2 to maximize the neutralization of SARS-CoV-2 (Wuhan-Hu-1, D614) and mutant isolates [6].
- the CR3022 epitope in RBD does not overlap with the ACE2 receptor binding motif [2-5]
- the CR3022 epitopes in SARS-CoV and SARS-CoV-2 RBD share 24 residues out of 28 (86% identity in amino acids) [4]
- Spike trimers stochastically exhibit their RBD in a “down” or “up” state [7, 8]
- the CR3022 antibody only accesses its epitope when at least two RBDs are in the “up” conformation and slightly-rotated [4, 5]
- CR3022 does not sterically prevent ACE2 binding to RBD [4, 5]
Yuan et al. used a microneutralization assay to show that CR3022 can neutralize SARS-CoV, but not SARS-CoV-2 [4]. On the contrary, Huo et al, showed that CR3022 does neutralize SARS-CoV-2, and their experimental data suggest that neutralization occurs by the destruction of the Spike protein in its prefusion conformation [5].
References
1. Jiang S. et al. 2020. Neutralizing antibodies against SARS-CoV-2 and other human coronaviruses. Trends Immunol. 41 (5):355-359.
2. Ter Muelen J.et al., 2006. Human monoclonal antibody combination against SARS coronavirus: synergy and coverage of escape mutants. PLos Med. 3(7):e237.
3. Tian X. et al., 2020. Potent binding of 2019 novel coronavirus spike protein by a SARS coronavirus-specific human monoclonal antibody. Emerging Microbes & Infections. 9(1):382-385.
4. Yuan M. et al., 2020. A highly conserved cryptic epitope in the receptor-binding domains of SARS-CoV-2 and SARS-CoV. Science. DOI: 10.1126/science.abb7269.
5. Huo J. et al., 2020. Neutralization of SARS-CoV-2 by destruction of the prefusion Spike. bioRxiv. DOI:10.1101/2020.05.05.079202.
6. Korber B. et al., 2020. Spike mutation pipeline reveals the emergence of a more transmissible form of SARS-CoV-2. bioRxiv. DOI:10.1101/2020.04.29.069054.
7. Walls A.C. et al., 2020. Structure, function, and antigenicity of the SARS-CoV-2 Spike glycoprotein. Cell. 181(2):281-292.e6.
8. Wrapp D. et al., 2020. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 367:1260-1263.