Anti-hIFN-α Neutralizing mAb
| Product | Unit size | Cat. code | Docs. | Qty. | Price | |
|---|---|---|---|---|---|---|
|
Anti-hIFNα-IgG Neutralizing recombinant IgG1 mAb against human IFN-α2 (clone H7WM116) |
Show product |
200 µg |
hifna-mab1-02
|
|
Neutralizing IgG monoclonal antibody to human interferon-alpha 2
Neutralizing monoclonal antibody against human IFN-α
Anti-hIFN-α-IgG (clone H7WM116) is a recombinant monoclonal antibody (mAb) able to neutralize human interferon alpha 2 (hIFN-α2). This mAb is expressed and produced in chinese hamster ovary (CHO) cells, ensuring reliability and lot-to-lot reproducibility. Thereby, common hybridoma-related drawbacks such as the generation of non-relevant mAbs containing aberrant light chains are avoided [1].
Anti-hIFN-α-IgG consists of the variable region of the Anti-hIFN-α mAb (clone H7WM116) and the engineered human IgG1 constant region. This antibody has been selected for its ability to efficiently neutralize the biological activity of hIFN-α2. It also reacts with various subtypes of the hIFN-α (see below, key features).
Key features:
- Potent and specific neutralization activity against hIFN-α2
- Reacts with hIFN-α1, hIFN-α2, hIFN-α5, hIFN-α8, hIFN-α14, hIFN-α16, hIFN-α17, and hIFN-α21
- Weakly reacts with hIFN-α4 and IFN-α10
- Does not react with hIFN-α6 or hIFN-α7
- Free from non-relevant mAbs found in hybridoma-based productions
- Provided azide-free
- Each lot is functionally tested
IFN-α is an important anti-viral cytokine that also has anti-proliferative and immunomodulatory functions [2, 3].
References:
1. Bradbury A. et al. 2018. When monoclonal antibodies are not monospecific: Hybridomas frequently express additional functional variable regions. mAbs, 10(4), 539–546.
2. Schreiber G. 2017. The molecular basis for differential type I interferon signaling. J. Biol. Chem. 292:7285-94.
3. McNab F. et al., 2015. Type I interferons in infectious disease. Nat Rev Immunol. 15(2):87-103.
Specifications
Target: Human interferon-alpha 2 (IFN-α2)
Specificity: Reacts with human IFN-α1, IFN-α2, IFN-α5, IFN-α8, IFN-α14, IFN-α16, IFN-α17 and IFN-α21. Very weakly reacts with human IFN-α4 and IFN-α10. It does not react with IFN-α6 or IFN-α7.
Clone: H7WM116
Source: CHO cells
Isotype: Human IgG1, kappa
Control: Anti-β-Gal-hIgG1
Formulation: 0.2 µm filtered solution in a sodium phosphate buffer with saccharose, glycine, and stabilizing agents
Purity: Purified by affinity chromatography with protein G
Applications: Blocking & Neutralization
Quality control:
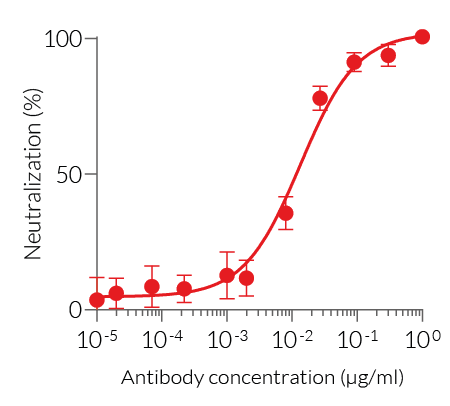
- This product have been validated using neutralization cellular assays.
- The complete sequence of the antibody has been verified.
- The absence of bacterial contamination (e.g. lipoproteins and endotoxins) has been confirmed using HEK-Blue™ TLR2 and HEK‑Blue™ TLR4 cells.
Contents
- 200 µg of purified Anti-hIFN-α-IgG antibody, provided azide-free and lyophilized.
![]() The product is shipped at room temperature.
The product is shipped at room temperature.
![]() Store lyophilized antibody at -20 °C.
Store lyophilized antibody at -20 °C.
![]() Lyophilized product is stable for at least 1 year.
Lyophilized product is stable for at least 1 year.
![]() Avoid repeated freeze-thaw cycles.
Avoid repeated freeze-thaw cycles.
Details
Type I interferons, in particular interferon-alpha (IFN-α) and interferon beta (IFN-β), play a vital role in host resistance to viral infections [1, 2]. The type I IFN family is a multi-gene cytokine family that encodes 13 partially homologous IFN-α subtypes in humans (14 in mice), a single IFN-β, and several poorly defined single gene products (IFN-ɛ, IFN-τ, IFN-κ, IFN-ω, IFN-δ, and IFN-ζ) [1, 2]. IFN-α and IFN-β are the best-defined and most broadly expressed type I IFNs [2].
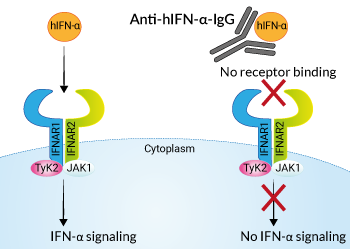
IFN-β and all of the IFN-α subtypes bind to a heterodimeric transmembrane receptor composed of the subunits IFNAR1 and IFNAR2 which are associated with the tyrosine kinases Tyk2 and Jak1 (Janus kinase 1) respectively. These kinases phosphorylate STAT1 and STAT2 which then dimerize and interact with IFN regulatory factor 9 (IRF9), leading to the formation of the ISGF3 complex. ISGF3 binds to IFN-stimulated response elements (ISRE) in the promoters of IFN-stimulated genes (ISG) to regulate their expression [1-3].
1. Schreiber G. 2017. The molecular basis for differential type I interferon signaling. J. Biol. Chem. 292:7285-94.
2. McNab F. et al., 2015. Type I interferons in infectious disease. Nat Rev Immunol. 15(2):87-103.
3. Theofilopoulos A. et al., 2005. Type I interferons (alpha/beta) in immunity and autoimmunity. Annu Rev Immunol. 23:307-36.