Human TLR3 Reporter HEK293 Cells (NF-κB)
| Product | Unit size | Cat. code | Docs. | Qty. | Price | |
|---|---|---|---|---|---|---|
|
HEK-Blue™ hTLR3 Cells Human TLR3 expressing HEK293 reporter cells (NF-κB pathway) |
Show product |
3-7 x 10e6 cells |
hkb-htlr3
|
|
||
|
HEK-Blue™ hTLR3 vial Additional cell vial |
Show product |
3-7 x 10e6 cells |
hkb-htlr3-av
|
Notification:
Reference #hkb-htlr3-av can only be ordered together with reference #hkb-htlr3.
NF-κB–SEAP reporter HEK293 cells expressing human TLR3
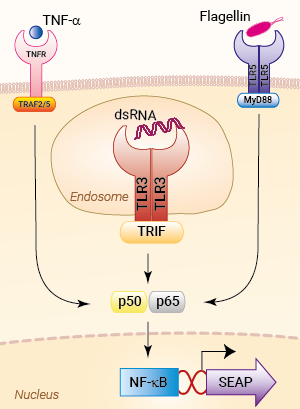
Signaling pathways in HEK-Blue™ hTLR3 cells
HEK-Blue™ hTLR3 cells were engineered from the human embryonic kidney HEK293 cell line to study the human Toll-like receptor 3 (TLR3)-dependent NF-κB response . This important pattern recognition receptor (PRR) recognizes double-stranded (ds)RNA, a hallmark of viral replication, and triggers antiviral NF-κB and IRF& immune responses [1].
Description
HEK-Blue™ hTLR3 cells feature the stable expression of the human TLR3 gene as well as an inducible reporter gene for SEAP (secreted embryonic alkaline phosphatase). SEAP levels produced upon TLR3 stimulation can be readily determined by performing the assay in HEK-Blue™ Detection , a cell culture medium that allows for real-time detection of SEAP. Alternatively, SEAP activity may be monitored using QUANTI-Blue™, a SEAP detection reagent.
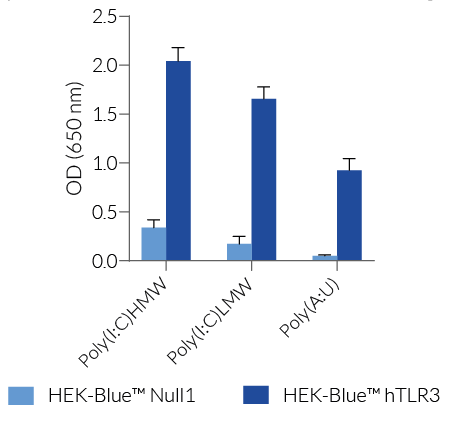
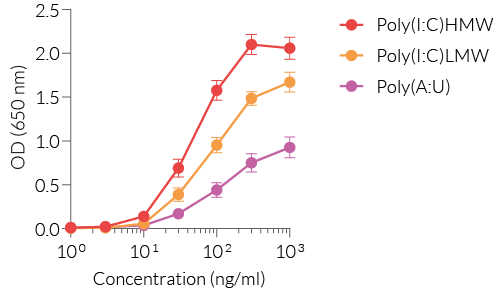
HEK-Blue™ hTLR3 cells are highly responsive to synthetic analogs of dsRNA. They show potent NF-κB response upon incubation with TLR3-specific ligands , such as Poly(I:C) (polyinosinic-polycytidylic acid) or Poly(A:U) (polyadenylic–polyuridylic acid), when compared to their parental cell line HEK-Blue™ Null1 (see figures).
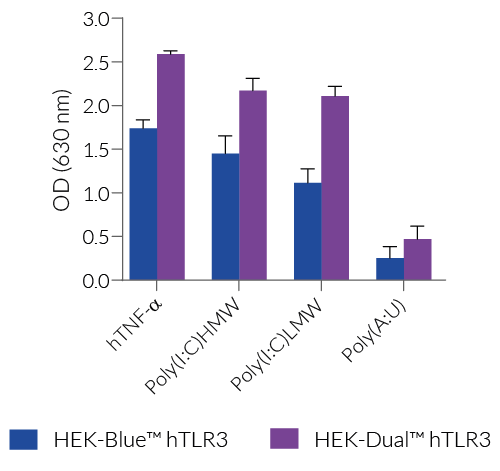
As TLR3 triggers both the NF-κB and IRF signaling pathways, InvivoGen also offers HEK-Dual™ hTLR3 cells; a cell line expressing a dual reporter system comprising an NF-κB-inducible SEAP and an IRF-inducible-Lucia luciferase (learn more here).
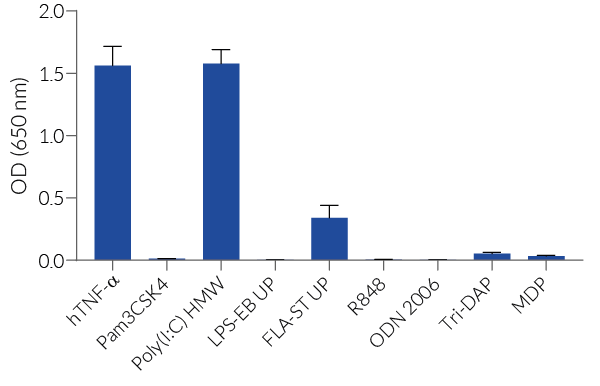
Of note, HEK293 cells express endogenous levels of various PRRs, including TLR3 and TLR5, and therefore might respond to their cognate ligands (see figures).
Key features
- Stable expression of human TLR3
- Strong response to dsRNA and analogs
- Distinct monitoring of TLR3-dependent NF-κB activation by assessing the SEAP activities
Applications
- Defining the role of TLR3-dependent NF-κB signaling pathway
- Screening for novel TLR3 agonists and inhibitors in comparison with their parental cell line HEK-Blue™ Null1
References
1. Chen Y, et al., 2021. Toll-like receptor 3 (TLR3) regulation mechanisms and roles in antiviral innate immune responses. J Zhejiang Univ Sci B.;22(8):609-632.
Back to the topSpecifications
Antibiotic resistance: Blasticidin, Zeocin®
Growth medium: DMEM, 4.5 g/l glucose, 2 mM L-glutamine, 10% (v/v) fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin, 100 μg/ml Normocin™
Quality Control:
- Stable expression of human (h)TLR3 has been verified by RT-qPCR and functional assays.
- The activation of NF-κB/AP1 upon TLR3 stimulation has been verified using functional assays.
- The stability for 20 passages, following thawing, has been verified.
- These cells are guaranteed mycoplasma-free.
Note: HEK293 cells express endogenous levels of TLR3, TLR5, and NOD1.
The appropriate parental cell line for HEK-Blue™ hTLR3 cells is HEK-Blue™ Null1.
Contents
- 1 vial containing 3-7 x 106 cells
- 1 ml of Blasticidin (10 mg/ml)
- 1 ml of Zeocin® (100 mg/ml)
- 1 ml of Normocin™ (50 mg/ml)
- 1 pouch of HEK-Blue™ Detection (cell culture medium for real-time detection of SEAP)
![]() Shipped on dry ice (Europe, USA, Canada, and some areas in Asia)
Shipped on dry ice (Europe, USA, Canada, and some areas in Asia)
Details
Toll-Like Receptor 3
In humans, four Toll-Like Receptor (TLR) family members TLR3, TLR7, TLR8, and TLR9 are specialized in sensing viral-derived components and are mainly found in the endosome. Among these, TLR3 recognizes double-stranded (ds)RNA, a hallmark of viral replication, and triggers antiviral immune responses [1]. TLR3 is expressed in myeloid dendritic cells, macrophages, as well as non-immune cells [2].
TLR3 signaling
TLR3 activation upon viral infection involves several steps, including translocation of TLR3 from the ER (endoplasmic reticulum) via the Golgi to the endosome, proteolytic cleavage and dimerization of TLR3, and finally receptor-ligand binding [3]. In order to start the signaling cascade, activated TLR3 recruits the adaptor protein TRIF (TIR domain-containing adapter-inducing interferon-β). TRIF binds to TRAF3 (TNF receptor-associated factor 3), which then recruits TBK1 (TANK-binding kinase 1) and IKKε (IκB kinase ε), thus activating the transcription factor IRF3 (interferon regulatory factor 3) and stimulating the production of type I IFNs (interferons). Additionally, TRIF interacts with TRAF6 and RIP1 (kinase receptor-interacting protein 1). RIP1 in turn binds to TAK1 (transforming growth factor β-activated kinase 1) and IKK. TAK1 phosphorylates IKKα and IKKβ, leading to the phosphorylation of IκB, the NF-κB inhibitor. Ultimately, this leads to the release and translocation of NF-κB into the nucleus and the induction of pro-inflammatory cytokines [2,4].
Pathology
Given its important role in dsRNA recognition, TLR3 signaling has been intensively studied. Various TLR3-agonists, such as the synthetic dsRNA analog Poly(I:C) are being used in vaccine development and cancer therapy [4]. Yet, recent studies have indicated that TLR3 may act as a double-edged sword by showing both protective and damaging functions in the context of some human viral infections [3,5]. Moreover, rare mutations in TLR3 have been associated with viral susceptibility; specifically, infections with HSV-1 (herpes simplex virus 1), influenza, and SARS-Co-V2 have been linked to pathogenic germline variants in TLR3 pathway genes [2]. Understanding the TRIF-dependent TLR3 pathway may be essential for the establishment of specific therapeutic approaches to diminish TLR3-driven disease and exploit its protective functions [3].
References
1. Manuela Sironi, et al., 2012. A Common Polymorphism in TLR3 Confers Natural Resistance to HIV-1 Infection. J Immunol 15; 188 (2): 818–823.
2. Aluri, J, et al., 2021. Toll-Like Receptor Signaling in the Establishment and Function of the Immune System. Cells, 10, 1374.
3. Chen Y, et al., 2021. Toll-like receptor 3 (TLR3) regulation mechanisms and roles in antiviral innate immune responses. J Zhejiang Univ Sci B.;22(8):609-632.
4. Komal A, et al., 2021. TLR3 agonists: RGC100, ARNAX, and poly-IC: a comparative review. Immunol Res. 69(4):312-322.
5. Perales-Linares R, Navas-Martin S. 2013. Toll-like receptor 3 in viral pathogenesis: friend or foe? Immunology.;140(2):153-67.