QS-21 (STIMULON®) VacciGrade™
| Product | Unit size | Cat. code | Docs. | Qty. | Price | |
|---|---|---|---|---|---|---|
|
QS-21 VacciGrade™ Saponin-based vaccine adjuvant (from plant cell culture) |
Show product |
5 mg 25 mg (5 x 5 mg) 1 mg |
vac-qs21-5
|
|
Saponin vaccine adjuvant formulation of STIMULON® QS-21 from cultured plant cells – Sterile
QS-21 extraction from tree bark and cultured plant cell
(click to enlarge)
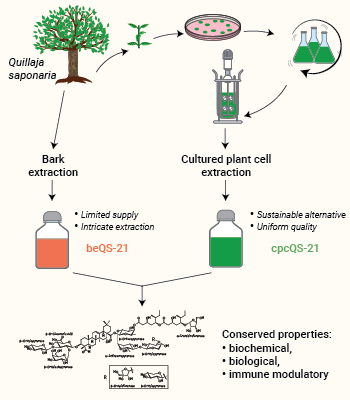
InvivoGen provides QS-21 VacciGrade™, a sterile and potent vaccine adjuvant obtained from plant cell culture of authenticated native Quillaja saponaria trees [1]. This innovative cultured plant cell production of QS-21 (cpcQS-21) offers a uniform quality and a sustainable alternative to conventional QS-21 extracted from the tree bark (beQS-21). Indeed, the limited cultivation of Chilean soapbark trees Quillaja saponaria, coupled to the intricate and costly bark extraction, makes conventional QS-21 (STIMULON® beQS-21) a scarce and expensive adjuvant.
Importantly, STIMULON® cultured plant cell QS-21 and STIMULON® bark extract QS-21 feature conserved chemical, biological, and immune modulatory properties [1].
QS-21 is a potent vaccine adjuvant and a key component of several licensed human vaccines. Notably, STIMULON® beQS-21 is used in adjuvant systems such as AS01®, a liposomal mixture of QS-21 and a TLR4 agonist, 3-O-desacyl-4'-monophosphoryl lipid A (MPL) [1, 2]. MPL is a detoxified derivative of lipopolysaccharide from Salmonella minnesota. QS-21-based vaccines induce humoral and Th1-type cellular immune responses [1, 3, 4]. QS-21 has been proposed to act primarily on antigen-presenting cells, enhancing antigen uptake and presentation by dendritic cells. Moreover, it triggers the NLRP3 inflammasome-dependent release of IL-1β and IL-18 [4, 5].
Key features
- Potent vaccine adjuvant
- Strong inducer of antibody-based and cell-mediated immune responses
- Natural and sustainable source
- Chemically and functionally equivalent to bark-extracted QS-21
- Sterile
- Batch-to-batch consistency
- Highly pure (≥ 96%)
VacciGrade™ is a high-quality pre-clinical grade.
QS-21VacciGrade™ is for research use only, and not for human or veterinary use. For your clinical or commercial needs, cGMP cultured plant cell QS-21 with identical biophysical properties is available from SaponiQx Inc.
Note: QS-21 VacciGrade™ is a sterile formulation of SaponiQx's proprietary STIMULON® cultured plant cell QS-21. STIMULON® is a registered trademark Agenus Inc., the parent company of SaponiQx Inc.
Note: AS01® is a registered trademark of GSK.
References:
1. Lv, X., et al., 2024. Chemical and biological characterization of vaccine adjuvant QS-21 produced via plant cell culture. iScience 109006.
2. Diderlaurent, A.M. et al., 2017. Adjuvant system AS01: helping to overcome the challenges of modern vaccines. Expert Rev. Vaccines 16(1):55.
3. Kensil, C.R., 1996. Saponins as vaccine adjuvants. Crit. Rev. Ther. Drug Carrier Syst. 13:1–55.
4. Lacaille-Dubois, M.A., 2019. Updated insights into the mechanism of action and clinical profile of the immunoadjuvant QS-21: A review. Phytomedicine. 60:152905.
5. Reinke, S., et al., 2020. Inflammasome-Mediated Immunogenicity of Clinical and Experimental Vaccine Adjuvants. Vaccines 8(3):554.
Specifications
Description: Saponin vaccine adjuvant, VacciGrade™
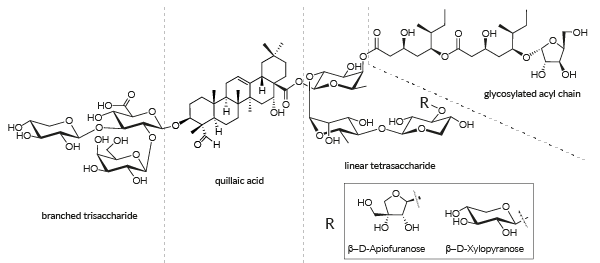
CAS Numbers: 141256-04-4 (QS-21 V1 isomer: β-D-Apiofuranose), 250643-56-2 (QS-21 V2 isomer: β-D-Xylopyranose)
Polarization of adaptive immune response: Th1 and Th17 cellular responses, Th2 humoral response
Formula: C92H148O46
Molecular weight: 1990.15 g/ml
Purity: ≥ 96% (HPLC)
Solubility: 1 mg/ml in phosphate buffer saline (PBS pH 6.8) or H2O
Note: If the product is resuspended in H2O, we recommend to sonicate and heat at 37°C for 5 to 10 min. Do not exceed 10 min incubation at 37°C as it may result in partial QS-21 hydrolysis.
Note: For stability and solubility purpose, we recommend to resuspend the product in PBS pH 6.8.
Dosing Guidelines: QS-21 VacciGrade™ is formulated for adjuvantation experiments in vivo, but it is also suitable for in vitro experiments. The resuspension solvent and method depends on your application. Please, refer to the literature. Examples can be found in:
- Lv, X. et al., 2024. Chemical and biological characterization of vaccine adjuvant QS-21 produced via plant cell culture. iScience 27, 109006.
- Marty-Roix, R. et al., 2016. Identification of QS-21 as an Inflammasome-activating Molecular Component of Saponin Adjuvants. J Biol Chem 291:1123.
Quality control:
- Sterility guaranteed
- The absence of bacterial contamination (lipoproteins & endotoxins) has been confirmed using HEK-Blue-Lucia™ hTLR2 and HEK-Blue-Lucia™ hTLR4 cells
- Endotoxin level < 10 EU/mg (measurement by kinetic chromogenic LAL assay)
Contents
QS-21 VacciGrade™ is provided as a lyophilized powder without excipients and is available in three quantities:
- 1 mg: vac-qs21-1
- 5 mg: vac-qs21-5
- 25 mg (5 x 5 mg): vac-qs21-25
![]() QS-21 VacciGrade™ is shipped at room temperature.
QS-21 VacciGrade™ is shipped at room temperature.
![]() Upon receipt, store at -20°C.
Upon receipt, store at -20°C.
![]() The product is stable for at least 1 year when properly stored.
The product is stable for at least 1 year when properly stored.
![]() Avoid repeated freeze-thaw cycles.
Avoid repeated freeze-thaw cycles.
Back to the top
VacciGrade™
VacciGrade™ is a high-quality pre-clinical grade. VacciGrade™ products are filter-sterilized (0.2 µm) and filled under strict aseptic conditions in a clean room*. The absence of bacterial contamination is assessed by a sterility test using a pharmacopeia-derived assay. The level of bacterial contaminants (endotoxins and lipoproteins) in each lot is verified using a LAL assay and/or a TLR2 and TLR4 reporter assay.
*Except for LPS VacciGrade™, which is prepared in a laminar flow hood dedicated to LPS.
Details
The saponin QS-21 isolated from the South American Quillaja Saponaria tree bark is one of the most potent adjuvants and is currently used in several licensed and exploratory human vaccines [1-3]. Notably, STIMULON® QS-21 isolated from bark extract (beQS-21) forms an integral part of the AS01® adjuvant. AS01® is a liposomal mixture of QS-21 and a TLR4 agonist, 3-O-desacyl-4'-monophosphoryl lipid A (MPL) [2, 3]. MPL is a detoxified derivative of lipopolysaccharide from Salmonella minnesota and TLR4 agonist. AS01® containing STIMULON® beQS-21 is a critical component of effective vaccines, including GSK’s Shingrix (Zoster Vaccine Recombinant, Adjuvanted) and Arexvy (Respiratory Syncytial Virus, Adjuvanted) [3]. QS-21 is also a component of Matrix-M adjuvant, which was included in the approved COVID-19 vaccine, NVX-CoV2373, and is used in the R21 malaria vaccine.
QS-21 structure
QS-21 is a mixture of two isomeric molecules, QS-21 β-D-Apiofuranose and QS-21 β-D-Xylopyranose, each having four domains: the triterpene quillaic acid, a branched trisaccharide, a linear tetrasaccharid, and a glycosylated acyl chain [1].
QS-21 adjuvancity
QS-21-based vaccines induce humoral and Th1-type cellular immune responses [4, 5]. Although the modes of action of QS-21 are not fully elucidated, it has been proposed that this triterpene saponin acts primarily on antigen-presenting cells (APCs).
QS-21 is endocytosed by dendritic cells in a cholesterol-dependent manner [6]. The carbohydrate domains and amphiphilic properties of QS-21 are thought to facilitate antigen uptake and presentation by dendritic cells [5].
QS-21, when formulated in liposomes, has been identified as an activator of the NLRP3 inflammasome in antigen-prensenting cells in vitro and in vivo, which results in the release of the potent proinflammatory cytokines IL-1β and IL-18 [7-9]. Importantly, the adjuvant potential of QS-21 via the NLRP3 inflammasome requires additional immunostimulatory substances, such as the TLR4 agonist MPLA [7]. Indeed, TLR priming is a prerequisite for robust inflammasome activation. However, currently available in vivo data for the role of NLRP3 inflammasome in adaptive immune responses warrants further research [7-9].
References:
1. Kensil, C.R., et al., 1991. Separation and characterization of saponins with adjuvant activity from Quillaja saponaria Molina cortex. J. Immunol. 146:431.
2. Diderlaurent, A.M. et al., 2017. Adjuvant system AS01: helping to overcome the challenges of modern vaccines. Expert Rev. Vaccines 16(1):55.
3. Lv, X., et al., 2024. Chemical and biological characterization of vaccine adjuvant QS-21 produced via plant cell culture. iScience 109006.
4. Kensil, C.R., 1996. Saponins as vaccine adjuvants. Crit. Rev. Ther. Drug Carrier Syst. 13:1–55.
5. Lacaille-Dubois, M.A., 2019. Updated insights into the mechanism of action and clinical profile of the immunoadjuvant QS-21: A review. Phytomedicine. 60:152905.
6. Lorent J.H., et al., 2014. The amphiphilic nature of saponins and their effects on artificial and biological membranes and potential consequences for red blood and cancer cells. Org Biomol Chem. 12(44):8803-22.
7. Marty-Roix, R., et al., 2016. Identification of QS-21 as an Inflammasome-activating Molecular Component of Saponin Adjuvants. J Biol Chem 291:1123.
8. Reinke, S., et al., 2020. Inflammasome-Mediated Immunogenicity of Clinical and Experimental Vaccine Adjuvants. Vaccines 8(3):554.
9. Detienne, S. et al., 2016. Central role for CD169(+) lymph node resident macrophages in the adjuvancity of the QS-21 component of AS01. Sci Rep. 6:39475.
Back to the top