NLRP1 Inflammasome Inducer – Val-boroPro
| Product | Unit size | Cat. code | Docs. | Qty. | Price | |
|---|---|---|---|---|---|---|
|
Val-boroPro NLRP1 activator (DPP8/9 inhibitor) |
Show product |
10 mg 50 mg |
tlrl-vbp-10
|
|
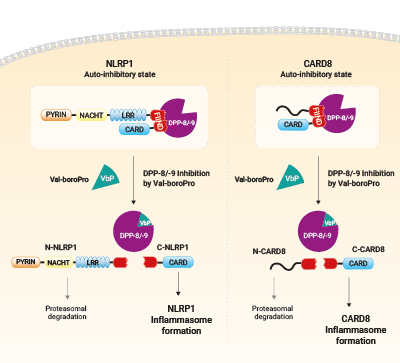
Activation of NLRP1 a CARD8 by Val-boroPro
Cellular dipeptidyl peptidase DPP-8 and DPP-9 function as endogenous inhibitor of the NLRP1 and CARD8 inflammasomes via both catalytic activity and scaffolding function. Pharmacological inhibiton of DPP-8/-9 with small molecule ValboroPro (VbP) abrogates the repression of NLRP1 and CARD8 inflammasomes which trigger their self-activation. It occurs following proteasomal degradation of NLRP1 and CARD8 N-terminal domain and release of their active C-terminal domain.
 InvivoGen also offers:
InvivoGen also offers:
NLRP1 inflammasome inducer
Val-boroPro (VbP, Talabostat), a dipeptide boronic acid, is a non-selective inhibitor of various dipeptidyl peptidases (DPPs) including DPP4, DPP8 and DPP9 [1]. It exhibits immunostimulatory properties, notably by disrupting the inhibitory interaction between DPP8/9 and the inflammasome sensor NLRP1 (NLR family member, pyrin domain containing 1). Hence, VbP is a potent inducer of NLRP1 inflammasome responses [1-3]. Moreover, this molecule has been shown to exert antitumor activity in vivo through the induction of cytokines and chemokines that participate in innate and adaptive immune responses [1-2].
Key features:
- Activator of the NLRP1 inflammasome (in human and mice)
- Pyroptosis inducer
- Non-selective inhibitor of serine dipeptidyl peptidases
- Each lot is functionally tested and highly pure (≥95%)
![]() Read our review on NLRP1 & NLRP3 inflammasome sensors
Read our review on NLRP1 & NLRP3 inflammasome sensors
References:
1. Okondo MC et al., 2017. DPP8 and DPP9 inhibition induces pro-caspase-1-dependent monocyte and macrophage pyroptosis. Nat Chem Biol. 2017;13(1):46-53.
2. Adams et al., 2004, PT-100, a Small Molecule Dipeptidyl Peptidase Inhibitor, Has Potent Antitumor Effects and Augments Antibody-Mediated Cytotoxicity via a Novel Immune Mechanism, Cancer Res 64 (15): 5471–5480.
3. Hollingsworth LR et al., 2021. DPP9 sequesters the C terminus of NLRP1 to repress inflammasome activation. Nature. 2021;592(7856):778-783.
Back to the top
Specifications
CAS number: 150080-09-4
Synonym: Talabostat, PT-100
Working concentration range: 10 - 100 µM for cell culture assays
Molecular weight: 310.18 g/mol
Solubility: 40 mg/ml in H2O
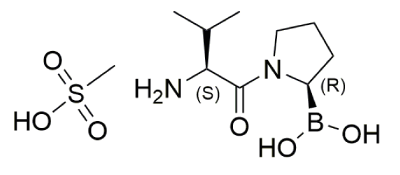
Formula: C9H19BN2O3.CH3SO3H
Quality control:
- Purity: ≥95% (HPLC)
- The biological activity has been confirmed using cellular assays.
- The absence of bacterial contamination (e.g. lipoproteins and endotoxins) is confirmed using HEK-Blue™ TLR2 and HEK-Blue™ TLR4 cells.
Contents
Val-boroPro is available in two quantities:
- 10 mg: tlrl-vpb-10
- 50 mg: tlrl-vpb-50
![]() Val-boroPro is shipped at room temperature.
Val-boroPro is shipped at room temperature.
![]() Upon receipt, store at -20°C.
Upon receipt, store at -20°C.
![]() Resuspended product is stable for at least 1 month at -20°C when properly stored.
Resuspended product is stable for at least 1 month at -20°C when properly stored.
![]() Avoid repeated freeze-thaw cycles.
Avoid repeated freeze-thaw cycles.
Details
VbP (also known as Talabostat, PT-100 or L-valinyl-L-boroproline) is a non-selective DPP inhibitor able to activate both the CARD8 (caspase recruitment domain-containing protein 8) and the NLRP1 inflammasomes [1]. It weakens the inhibitory complex between DPP9 active site and the bioactive C-terminus of NLRP1 by direct competition for DPP9’s catalytic residues. As a result, the single bound C-terminal NLRP1 is released. At the same time, the full length NLRP1 is displaced from the interaction, which leads to auto-proteolysis of its FIIND domain and to proteasomal degradation of the N-terminal domain [3]. The bioactive C-terminal CARD domain is released, activates Caspase-1 via oligomerization with ASC proteins, and subsequently, triggers pyroptosis [2-4].
Additionally, VbP exhibits potent antitumor activity in vivo through the induction of cytokines and chemokines activating the innate and adaptive immune system [2,4]. It also increases the effect of antitumor antibodies like Rituximab and Trastuzumab in xenograft models of human CD20+ B cell lymphoma and HER2+ colon carcinoma, respectively [4].
1. Sato T, et al., 2019. Hatano R, Iwao N, Ohnuma K, Morimoto C. DPP8 is a novel therapeutic target for multiple myeloma. Sci Rep. 2;9(1):18094.
2. Okondo MC, et al., 2017. DPP8 and DPP9 inhibition induces pro-caspase-1-dependent monocyte and macrophage pyroptosis. Nat Chem Biol. 13(1):46-53.
3. Hollingsworth LR, et al., 2021. DPP9 sequesters the C terminus of NLRP1 to repress inflammasome activation. Nature. 592(7856):778-783.
4. Adams et al., 2004. PT-100, a Small Molecule Dipeptidyl Peptidase Inhibitor, Has Potent Antitumor Effects and Augments Antibody-Mediated Cytotoxicity via a Novel Immune Mechanism, Cancer Res 64 (15): 5471–5480.
Chemical structure of Val-boroPro: