LFn-Rod
| Product | Unit size | Cat. code | Docs. | Qty. | Price | |
|---|---|---|---|---|---|---|
|
LFn-Rod T3SS Inner Rod protein fused to Lethal Factor - NLRC4 agonist |
Show product |
50 µg |
tlrl-rod
|
|
Inflammasome activation with Rod
Mouse NLRC4/NAIP Inflammasome Inducer
InvivoGen provides LFn-Rod, a model of the murine NLRC4/NAIP inflammasome agonist [1-3]. The Inner Rod protein is a component of the type III secretion systems (T3SS) of intracellular bacteria able to interact with NLRC4 via NAIP [1-5].
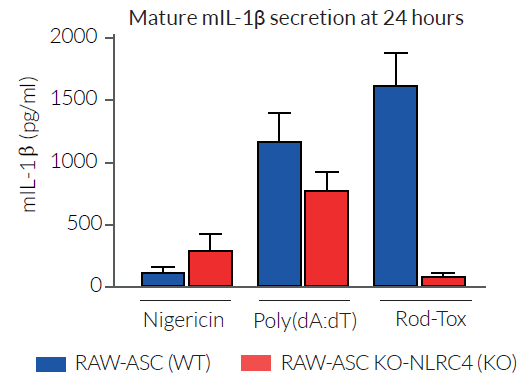
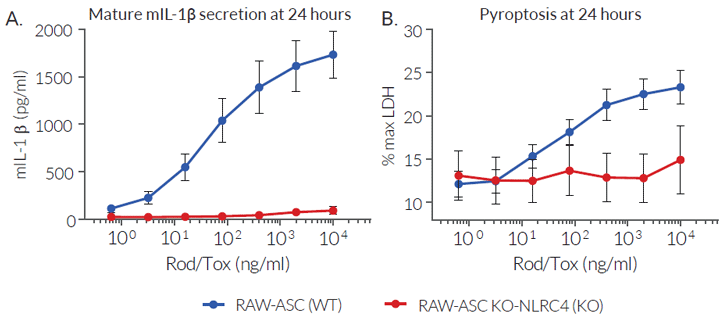
LFn-Rod is fused to the amino-terminal domain of B. anthracis lethal factor (LFn). This fusion system, when co-administred with the anthrax toxin’s protective antigen (PA), allows intracellular delivery of the bacterial ligand [6]. The combination of LFn-Rod with the anthrax protective antigen (PA) is named Rod-Tox [3]. Its ability to activate the murine NLRC4 inflammasome has been validated using RAW-ASC and RAW-ASC KO-NLRC4 cell lines.
 InvivoGen also offers:
InvivoGen also offers:
• LFn-Needle: human NLRC4 inducer
• RAW-ASC KO-GSDMD Cells: Inflammasome Test Cells
Key features:
- Inner Rod (PrgJ) from S. typhimurium T3SS
- Potent inducer of the murine NLRC4 inflammasome in vitro
- Produced in Sf9 insect cells
- Each lot is functionally tested
![]() Download our Practical guide on Inflammasomes.
Download our Practical guide on Inflammasomes.
References:
1. Zhao Y. et al., 2011. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature. 477(7366):596-600.
2. Kofoed E.M. & Vance R.E., 2011. Innate immune recognition of bacterial ligands by NAIPs determines inflammasome specificity. Nature. 477(7366):592-595.
3. Rauch I. et al., 2016. NAIP proteins are required for cytosolic detection of specific bacterial ligands in vivo. The Journal of Exp. Med. 213(5):657-665.
4. Zhao Y. et al., 2016. Genetic functions of the NAIP family of inflammasome receptors for bacterial ligands in mice. J Exp Med. 213(5):647-656.
5. Worrall L.J. et al., 2011. Structural overview of the bacterial injectisome. Curr Opin Microbiology. 14(1):3-8.
6. Ballard J.D. et al., 1996. Anthrax toxin-mediated delivery of a cytotoxic T-cell epitope in vivo. PNAS. 93(22):12531-12534.
Specifications
Protein construction: T3SS Inner Rod (PrgJ) protein [M1-S101] fused to the amino-terminal domain [A34-R296] of anthrax toxin’s lethal factor (LFn) protein (N-terminal).
Accession sequence: WP_00002043 (Inner Rod; PrgJ sequence)
Species: Salmonella typhimurium
Source: Sf9 insect cells
Tag: N-terminal poly-histidine (6 x His)
Total protein size: 384 a.a. (secreted form)
Molecular weight: ~ 48 kDa (SDS-PAGE gel)
Purification: Ni2+ affinity chromatography
Purity: >90% (SDS-PAGE)
Quality control:
- The protein has been validated using functional assays.
- The absence of bacterial contamination (e.g. lipoproteins and endotoxins) has been confirmed using HEK-Blue™ TLR2 and HEK-Blue™ TLR4 cellular assays.
Back to the top
Contents
Note: B. anthracis protective antigen (PA) is not provided
- 50 μg of lyophilized LFn-Rod protein
- 1.5 ml of endotoxin-free water
![]() The product is shipped at room temperature.
The product is shipped at room temperature.
![]() Lyophilized protein should be stored at -20 ̊C up to 6 months.
Lyophilized protein should be stored at -20 ̊C up to 6 months.
![]() Resuspended protein is stable up to 6 months when stored at -20°C
Resuspended protein is stable up to 6 months when stored at -20°C
Avoid repeated freeze-thaw cycles.
Back to the topDetails
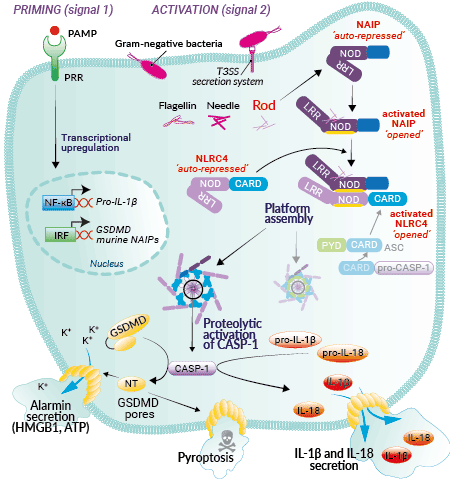
NLRC4 BACKGROUND:
The NLRC4 (aka Ipaf) inflammasome is an intracellular multi-protein complex that plays a central role in innate immunity [1,2]. It is activated by a two-step process; a first signal (‘priming’) is provided by microbial molecules such as TLR ligands, while the second signal is provided by intracellular bacterial molecules such as Flagellin from the motility apparatus, or Inner Rod and Needle proteins from the bacterial type III or IV secretion systems (T3SS or T4SS). NLCR4 is an indirect sensor: it interacts with NAIPs (NLR family apoptosis inhibitory proteins) that directly bind to Flagellin, Needle, and Inner Rod. While a single NAIP operates upstream of NLRC4 in humans and recognizes each of these activators [3], multiple NAIPs have been described in mice with different affinities for each molecule [4-7]. The NLRC4 inflammasome appears to protect mucosal barriers, such as the lung, stomach, and intestine, from invading bacteria [2].
References:
1. Platnich J.M. & Muruve D.A., 2019. NOD-like receptors and inflammasomes: A review of their canonical and non-canonical signaling pathways. Arch Biochem Biophys. 670:4-14.
2. Bauer R. & Rauch I., 2020. The NAIP/NLRC4 inflammasome in infection and pathology. Mol Aspects Med. 76:100863.
3. Yang J. et al., 2013. Human NAIP and mouse NAIP1 recognize bacterial type III secretion needle protein for inflammasome activation. PNAS. 110(35):14408-14413.
4. Zhao Y. et al., 2011. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature. 477(7366):596-600.
5. Rauch I. et al., 2016. NAIP proteins are required for cytosolic detection of specific bacterial ligands in vivo. The Journal of Exp. Med. 213(5):657-665.
6. Zhao Y. et al., 2016. Genetic functions of the NAIP family of inflammasome receptors for bacterial ligands in mice. J Exp Med. 213(5):647-656.
7. Kofoed E.M. & Vance R.E., 2011. Innate immune recognition of bacterial ligands by NAIPs determines inflammasome specificity. Nature. 477(7366):592-595.