A549-Dual™ Cells for SARS-CoV-2 studies
| Product | Unit size | Cat. code | Docs. | Qty. | Price | |
|---|---|---|---|---|---|---|
|
A549-Dual™ hACE2-TMPRSS2 Cells Reporter Human Lung Carcinoma |
Show product |
3-7 x 10e6 cells |
a549d-cov2r
|
|
||
|
A549-Dual™ hACE2-TMPRSS2 vial Additional cell vial |
Show product |
3-7 x 10e6 cells |
a549d-cov2r-av
|
|||
|
A549-Dual™ KO-MDA5 hACE2-TMPRSS2 Cells MDA5 knockout - Reporter Human Lung Carcinoma |
Show product |
3-7 x 10e6 cells |
a549d-komda5-cov2r
|
|
||
|
A549-Dual™ KO-MDA5 hACE2-TMPRSS2 vial Additional cell vial |
Show product |
3-7 x 10e6 cells |
a549d-komda5-cov2r-av
|
|||
|
A549-Dual™ KO-RIG-I hACE2-TMPRSS2 Cells RIG-I Knockout - Reporter Human Lung Carcinoma |
Show product |
3-7 x 10e6 cells |
a549d-korigi-cov2r
|
|
||
|
A549-Dual™ KO-RIG-I hACE2-TMPRSS2 vial Additional cell vial |
Show product |
3-7 x 10e6 cells |
a549d-korigi-cov2r-av
|
Notification:
Reference #a549d-cov2r-av can only be ordered together with reference #a549d-cov2r.
Reference #a549d-komda5-cov2r-av can only be ordered together with reference #a549d-komda5-cov2r.
Reference #a549d-korigi-cov2r-av can only be ordered together with reference #a549d-korigi-cov2r.
Dual reporter cells expressing the SARS-CoV-2 receptors ACE2 & TMPRSS2
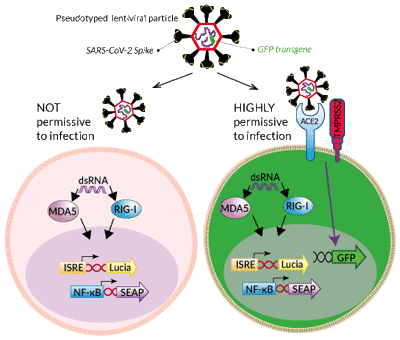
Reporter systems in A549-Dual™-derived cells
InvivoGen offers a new series of A549-Dual™ cell lines, specifically designed for COVID-19 studies:
— A549-Dual™ hACE2-TMPRSS2 cells
— A549-Dual™ KO-MDA5 hACE2-TMPRSS2 cells
— A549-Dual™ KO-RIG-I hACE2-TMPRSS2 cells
These cells derive from the human A549 lung carcinoma cell line. They stably overexpress the genes encoding for the SARS-CoV-2 receptors, human ACE2, and TMPRSS2.
These cells also express two inducible reporter genes, allowing the concomitant study of the IRF and NF-κB pathways, by monitoring the Lucia luciferase and SEAP (secreted embryonic alkaline phosphatase) activities, respectively. In addition, these cells either express MDA5 (Melanoma Differentiation Associated gene 5) and RIG-I (Retinoic Acid Inducible protein 1) endogenously or are knockout for each gene, individually. MDA5 and RIG-I are sensors of viral RNA that have been reported to participate in the immune response to SARS-CoV-2 infection [1-3].
The A549 cell line is commonly used for the study of respiratory infections. SARS-CoV-2, the causative agent of coronavirus disease-19 (COVID-19), gains entry into the human lung epithelium through the interaction of the virus Spike protein with the host ACE2 and TMPRSS2 receptors [3-5]. A549 cells express negligible levels of ACE2 and no TMPRSS2 and thus are poorly permissive to infection by SARS-CoV-2 or Spike pseudotyped lentiviral particles [4-6, in-house data]. To increase their permissivity, A549-Dual™ cells have been stably transfected with the human ACE2 and TMPRSS2 genes. In contrast to A549-Dual™ cells, A549-Dual™ hACE2-TMPRSS2 cells are highly permissive to Spike pseudotyped lentiviral particles as visualized by the expression of the lentiviral GFP transgene (see Figures).
Key Features:
- Overexpression of human ACE2 and TMPRSS2
- Highly permissive to SARS-CoV-2 Spike pseudotyped lentiviral particles
- Readily assessable Lucia luciferase and SEAP reporter activity for the IRF and NF-κB activation, respectively
- Endogenous or knockout expression of human MDA5 or human RIG-I
Applications:
- Study of SARS-CoV-2 RNA sensing pathways
- Screening of small molecule inhibitors and/or neutralizing antibodies of the ACE2‑Spike interaction
- Screening of small molecule inhibitors and/or neutralizing antibodies of the TMPRSS2 surface protease
- Comparative studies of the effects of drugs targeting ACE2 and/or TMPRSS2 on the SARS-CoV-2 infection and cellular signaling outcomes
![]() Learn more about SARS-CoV-2 infection cycle, immune responses, and potential therapeutics.
Learn more about SARS-CoV-2 infection cycle, immune responses, and potential therapeutics.
References
1. Ying X. et al., 2021. MDA5 governs the innate immune response to SARS-CoV-2 in lung epithelial cells. Cell Reports. 34:108628.
2. Rebendenne A. et al., 2021. SARS-CoV-2 triggers MDA-5-dependent interferon response which is unable to control replication in lung epithelial cells. J. Virol. doi:10.1128/JVI.02415-20.
3. Wu J, et al., 2021. SARS-CoV-2 ORF9b inhibits RIG-I MAVS antiviral signaling by interrupting K63-linked ubiquitination of NEMO. Cell Reports. 34(7):108761.
4. Chen H. et al., 2020. SARS-CoV-2 activated lung epithelia cell proinflammatory signaling and leads to immune dysregulation in COVID-19 patients by single-cell sequencing. medRxiv: DOI 10.1101/2020.05.08.20096024.
5. Hoffmann M. et al., 2020. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 181:1-16.
6. Matsuyama S. et al., 2020. Enhanced isolation of SARS-CoV-2 by TMPRSS2-expressing cells. PNAS. 117(13):7001-7003.
Specifications
Growth medium: DMEM, 4.5 g/L glucose, 2 mM L-glutamine, 10% (v/v) heat-inactivated fetal bovine serum (FBS), 100 U/ml penicillin, 100 µg/ml streptomycin, 100 µg/ml Normocin™
Antibiotic resistance: Blasticidin, Hygromycin, Puromycin, and Zeocin®
Quality Control:
- ACE2 gene expression has been verified by RT-qPCR, FACS staining, and functional assays.
- TMPRSS2 gene expression has been verified by RT-qPCR, and functional assays.
- MDA5 knockout has been verified by RT-qPCR and functional assays.
- RIG-I knockout has been verified by RT-qPCR and functional assays.
- Lucia luciferase and SEAP reporter activities have been validated using functional assays.
- The stability for 20 passages, following thawing, has been verified.
- These cells are guaranteed mycoplasma-free.
Contents
Please note: Each cell line is sold separately. See TDS for the exact contents of each cell line.
- 3-7 x 106A549-Dual™ hACE2-TMPRSS2 cells, OR A549-Dual™ KO-MDA5 hACE2-TMPRSS2 cells, OR A549-Dual™ KO-RIG-I hACE2-TMPRSS2 cells in a cryovial or shipping flask
- 1 ml of Blasticidin (10 mg/ml)
- 1 ml of Hygromycin B Gold (100 mg/ml)
- 1 ml of Puromycin (10 mg/ml)
- 1 ml of Zeocin®(100 mg/ml)
- 1 ml of Normocin™ (50 mg/ml). Normocin™ is a formulation of three antibiotics active against mycoplasmas, bacteria, and fungi.
- 1 ml of QB reagent and 1 ml of QB buffer (sufficient to prepare 100 ml of QUANTI-Blue™ Solution, a SEAP detection reagent)
- 1 tube of QUANTI-Luc™ 4 Reagent, a Lucia luciferase detection reagent (sufficient to prepare 25 ml)
![]() Shipped on dry ice (Europe, USA, Canada, and some areas in Asia)
Shipped on dry ice (Europe, USA, Canada, and some areas in Asia)
Details
ACE2 AND TMPRSS2, CELL SURFACE SARS-COV-2 RECEPTORS:
ACE2 (angiotensin I-converting enzyme-2) and TMPRSS2 (transmembrane protease serine 2) play a critical role in the pathogenesis of COVID-19 by allowing viral entry into target cells (e.g. human lung epithelium). ACE2 and TMPRSS2 are cell-surface proteins that both interact with the virus Spike (S) protein [1,2-4]. ACE2 is mandatory for the binding of SARS-CoV-2 at the cell surface through its interaction with the Spike receptor-binding domain (RBD) [5]. Following this, TMPRSS2 cleaves the S protein into two functional subunits (S1 and S2), allowing virus-host membrane fusion, and the release of viral contents (e.g. RNA) into the cytosol [4-6]. Another protease, the Cathepsin L, also mediates cleavage of the S protein but it acts in the endosomes. Camostat is a clinically-proven inhibitor of TMPRSS2 and has been shown to inhibit SARS-CoV-2-S pseudotyped viral particles entry into primary human lung cells in a dose-dependent manner [2]. This observation demonstrates the critical implication of TMPRSS2 in SARS-CoV-2 infection and spread. Moreover, in lung cells that fail to express robust levels of Cathepsin L, the virus entry depends on a furin-mediated pre-cleavage of the S protein at the S1/S2 site, before subsequent TMPRSS2-mediated cleavage at the S2' site [7].
1. Chen H. et al., 2020. SARS-CoV-2 activated lung epithelia cell proinflammatory signaling and leads to immune dysregulation in COVID-19 patients by single-cell sequencing. medRxiv: DOI 10.1101/2020.05.08.20096024.
2. Hoffmann M. et al., 2020. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 181:1-16.
3. Birra D. et al., 2020. COVID 19: a clue from innate immunity. Immunologic Research. 68(3):161-168.
4. Matsuyama S. et al., 2020. Enhanced isolation of SARS-CoV-2 by TMPRSS2-expressing cells. PNAS. 117(13):7001-7003.
5. Zhou P. et al., 2020. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 579(7798):270-273.
6. Walls A.C. et al., 2020. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 181(2):281-292.e6.
7. Hoffman M. et al., 2020. A multibasic cleavage site in the Spike protein of SARS-CoV-2 is essential for infection of human lung cells. Molecular Cell. 78:1-6.




