Jurkat-Raji CD27/CD70 assay (Bio-IC™)
| Product | Unit size | Cat. code | Docs. | Qty. | Price | |
|---|---|---|---|---|---|---|
|
CD27/CD70 Bio-IC™ 2 cell lines (Jurkat & Raji) based Lucia luciferase reporter assay |
Show product |
3-7 x 10e6 cells (x2) |
rajkt-cd27
|
|
Antagonist screening assay for CD27/CD70 axis
InvivoGen offers a cellular assay specifically designed for screening antibody-, Fc-fusion protein-, or small-molecule antagonists of the CD27/CD70 immune checkpoint (IC) axis. This assay is comprised of:
Principle of CD27/CD70 cellular assay
(click to enlarge)
 InvivoGen also offers:
InvivoGen also offers:
• Agonist screening assay for CD27/CD70 axis
• Fc-hCD70 fusion protein
• hCD27-Fc fusion protein
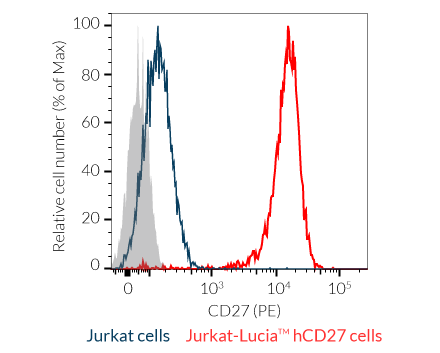
- Jurkat-Lucia™ hCD27: Reporter T cells
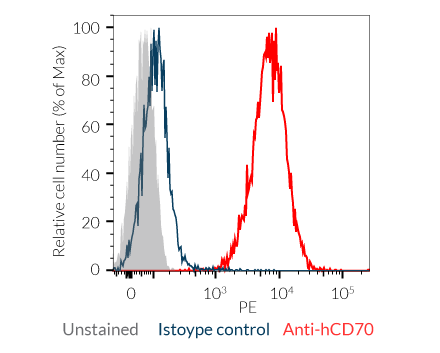
- Raji-Null: Antigen-presenting cells (APCs)
These paired cell lines allow the mimicking of the CD27-CD70-mediated co-stimulation between T cells and APCs.
CD27 is an immunostimulatory IC and a member of the TNFR (tumor necrosis factor receptor) superfamily. In humans, CD27 is constitutively expressed on most T cells, memory B cells, plasma cells, and Natural Killer cells. Expression of CD70 is tightly controlled and induced transiently in APCs following stimuli exposure, such as TFN-α, irradiation, or TLR agonists [1, 2]. The CD27–CD70 axis pair plays an important role in immune regulation. In concert with the T cell receptor (TCR) crosslinking, it contributes to efficient T cell activation, proliferation, survival, maturation of effector capacity, and T cell memory [1, 2].
Note: If you are interested in a model that allows the mimicking of an immune synapse through T cell receptor (TCR) crosslinking with [HLA::peptide] complexes, along with the CD27-CD70 costimulatory interaction, please contact us.
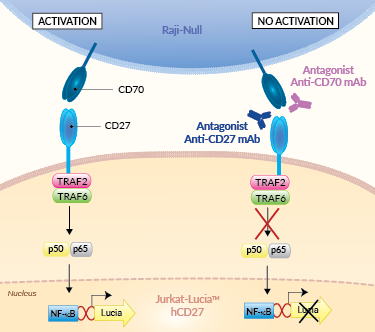
Assay principle:
This assay relies on the co-culture of Jurkat-Lucia™ hCD27 and Raji-Null cells:
– Jurkat-Lucia™ hCD27 cells stably express cell surface CD27, along with an NF-κB-inducible Lucia luciferase reporter gene.
– Raji-Null cells express the CD70 activating IC ligand.
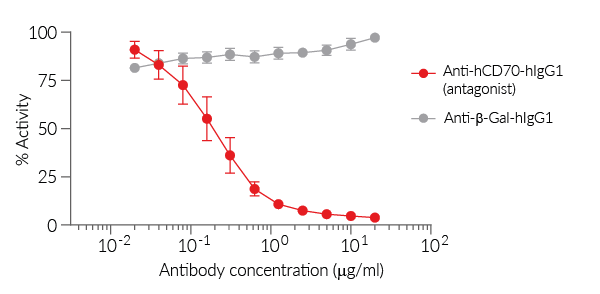
In the presence of a potent CD27 or CD70 antagonist, the CD27-CD70-mediated activation is blocked and there is no Lucia production. Activation of the reporter T cells can be readily measured using QUANTI-Luc™ 4 Lucia/Gaussia detection reagent (see Figures).
T-cell key features:
- Stable human CD27 surface expression
- Fully functional CD27 signaling pathway
- NF-κB-inducible Lucia luciferase reporter activity
APC key features:
- High surface expression of human CD70
InvivoGen also offers Jurkat-Lucia™ hCD27 cells without Raji-Null cells to measure the potency of agonists of the CD27/CD70 axis.
![]() Read our review on Immune Checkpoint Blockade
Read our review on Immune Checkpoint Blockade
![]() Learn more about Immune Checkpoint Antibodies.
Learn more about Immune Checkpoint Antibodies.
References:
1. Jacobs, J. et al. 2015. CD70: An emerging target in cancer immunotherapy. Pharmacol Ther 155, 1-10.
2. Buchan S.L, et al. 2018. The immunobiology of CD27 and OX40 and their potential as targets for cancer immunotherapy. Blood 131(1):39-48.
Specifications
Growth medium: IMDM, 2 mM L-glutamine, 25 mM HEPES, 10% (v/v) heat-inactivated fetal bovine serum (FBS), 100 U/ml penicillin, 100 µg/ml streptomycin, 100 µg/ml Normocin™
Antibiotic resistance:
- Jurkat-Lucia™ hCD27 cells: Blasticidin and Zeocin®
- Raji-Null cells: Blasticidin
Quality Control:
- Human CD27 and CD70 expression is confirmed by flow cytometry.
- Reporter activity is validated following the co-culture of the two cell lines.
- The stability for 20 passages following thawing is verified.
- The cells are guaranteed mycoplasma-free.
Contents
Please note: Both cell lines are sold together.
- 3-7 x 106 Jurkat-Lucia™ hCD27 cells
- 3-7 x 106 Raji-Null cells
- 1 ml of Blasticidin (10 mg/ml)
- 1 ml of Zeocin® (100 mg/ml)
- 1 ml of Normocin™ (50 mg/ml). Normocin™ is a formulation of three antibiotics active against mycoplasmas, bacteria, and fungi.
- 1 tube of QUANTI-Luc™ 4 Reagent, a Lucia luciferase detection reagent (sufficient to prepare 25 ml)
![]() Shipped on dry ice (Europe, USA, Canada)
Shipped on dry ice (Europe, USA, Canada)
FAQ Cell Lines
 Any questions about our cell lines ? Visit our frequently asked questions page
Any questions about our cell lines ? Visit our frequently asked questions page
Back to the top
Details
The CD27/CD70 immune checkpoint
The CD27–CD70 axis plays an important role in immune regulation and is considered an immune checkpoint (IC).
The cluster of differentiation CD27 is a member of the TNFR (tumor necrosis factor receptor) superfamily. In humans, CD27 is constitutively expressed on most T cells, memory B cells, plasma cells, and Natural Killer cells [1].
The Cluster of Differentiation CD70 (aka CD27L, TNFSF7) is also a member of the TNFR family and is known as the sole ligand for CD27. Expression of CD70 is tightly controlled and induced transiently in antigen-presenting cells (APCs) following stimuli exposure, such as TFN-α, irradiation, or TLR agonists [2, 3].
The CD27 and CD70 interaction triggers the TRAF2-TRAF6-NF-kB signaling pathway, and in concert with the T cell receptor (TCR) crosslinking, contributes to efficient T cell activation, proliferation, survival, maturation of effector capacity, and T cell memory [2-4].
The CD27/CD70 immune checkpoint in cancer
CD27 is expressed in various types of hematologic cancers. In leukemia, CD27 signaling leads to the induction of different pathways, supporting stemness, tumor cell proliferation, and self-renewal [1]. The efficacy of targeting the CD27/CD70 axis with an agonistic CD27 mAbs was shown in preclinical models of lymphoma, renal cell carcinoma (RCC), breast cancer, and sarcoma [5]. A human mAb directed at CD27 named varlilumab (also CDX-1127, 1F5) has entered clinical trials. It can activate CD27-positive T cells while mediating the killing of CD27-expressing tumor cells [5, 6]. Also, it has successfully completed a phase I/II dose escalation and cohort expansion study (NCT02335918) in combination with nivolumab in different solid malignancies. Further phase I trials with various combinations as well as phase II trials are still ongoing in renal cell carcinoma, squamous cell carcinoma of the head and neck, ovarian and colorectal cancers, and glioblastoma [5].
Constitutive overexpression of CD70 has been described in a range of solid and hematological malignancies, whereby tumor cells have hijacked the CD27-CD70 signaling pathway to facilitate immune evasion in the tumor microenvironment (TME) [7]. They do this by increasing the amount of suppressive regulatory T cells (Tregs), inducing caspase-dependent apoptosis of T cells, and skewing T cells towards exhaustion, to ensure they circumvent the immune response [2]. Additionally, the presence of CD70 on cancer stem cells is a predictive marker for metastasis and poor prognosis [8]. Exploiting CD70-targeting in cancer patients could help eliminate the CD70-expressing cancer cell populations and abrogate the tumor-promoting mechanisms by the CD70-CD27 signaling axis, both in the early stage and advanced disease. Combinatorial approaches with anti-CD70 targeting therapies have proven their potential in both preclinical and clinical settings, using antibody-mediated therapy, chemotherapy, and Chimeric Antigen Receptor (CAR)-T cell therapy [1].
References:
1. Flieswasser, T., Van den Eynde, A., Van Audenaerde, J. et al. 2022. The CD70-CD27 axis in oncology: the new kids on the block. J Exp Clin Cancer Res 41, 12.
2. Jacobs, J. et al. 2015. CD70: An emerging target in cancer immunotherapy. Pharmacol Ther 155, 1-10.
3. Buchan S.L, et al. 2018. The immunobiology of CD27 and OX40 and their potential as targets for cancer immunotherapy. Blood 131(1):39-48.
4. Sanborn RE, et al., 2022. Safety, tolerability and efficacy of agonist anti-CD27 antibody (varlilumab) administered in combination with anti-PD-1 (nivolumab) in advanced solid tumors. J Immunother Cancer. 10(8):e005147.
5. Starzer AM, Berghoff AS., 2020. New emerging targets in cancer immunotherapy: CD27 (TNFRSF7). ESMO Open. 4 (Suppl 3):e000629.
6. Vitale LA, et al., 2012. Development of a human monoclonal antibody for potential therapy of CD27-expressing lymphoma and leukemia. Clin Cancer Res. 18(14):3812-21
7. Jacobs, J. et al. 2018. Unveiling a CD70-positive subset of cancer-associated fibroblasts marked by pro-migratory activity and thriving regulatory T cell accumulation. Oncoimmunology 7, e1440167.
8. Liu, L. et al. 2018. Breast cancer stem cells characterized by CD70 expression preferentially metastasize to the lungs. Breast Cancer 25, 706-716.