Jurkat Reporter Cells (NFAT)
| Product | Unit size | Cat. code | Docs. | Qty. | Price | |
|---|---|---|---|---|---|---|
|
Jurkat-Lucia™ NFAT Cells NFAT-Luc Reporter T Lymphocytes |
Show product |
3-7 x 10e6 cells |
jktl-nfat
|
|
||
|
Jurkat-Lucia™ NFAT vial Additional cell vial |
Show product |
3-7 x 10e6 cells |
jktl-nfat-av
|
Notification: Reference #jktl-nfat-av can only be ordered together with reference #jktl-nfat.
NFAT–Lucia reporter T Lymphocytes
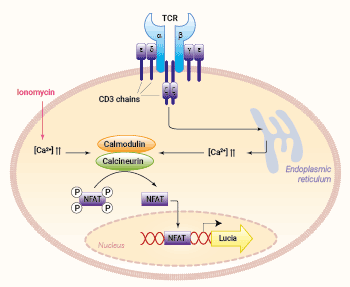
Jurkat-Lucia™ NFAT Cells signaling pathway
Jurkat-Lucia™ NFAT cells are derived from the human T lymphocyte Jurkat cell line through the stable integration of an NFAT-inducible reporter gene encoding the Lucia luciferase. As a result, these cells allow the study of the nuclear factor of activated T cells (NFAT) pathway, by measuring the Lucia activity in the cell culture supernatant using QUANTI-Luc™ 4 Lucia/Gaussia, a Lucia luciferase detection reagent.
Jurkat cells naturally express functional NFAT proteins, a family of transcription factors involved in T cell activation, differentiation, and self-tolerance [1, 2]. NFAT activation is controlled by calcium influx upon T cell receptor (TCR) stimulation [1, 2]. Several therapeutic approaches have focused on targeting the NFAT signaling to control T cell responses in autoimmune diseases and graft rejection [1].
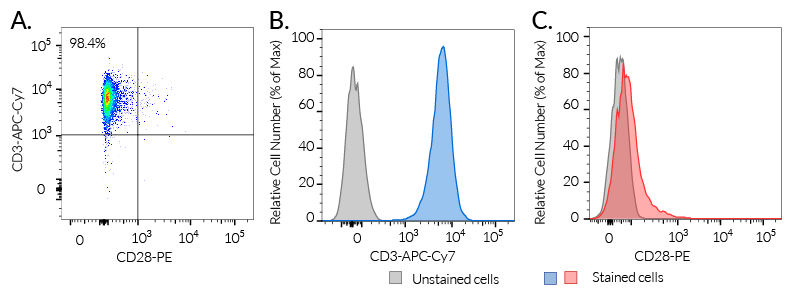
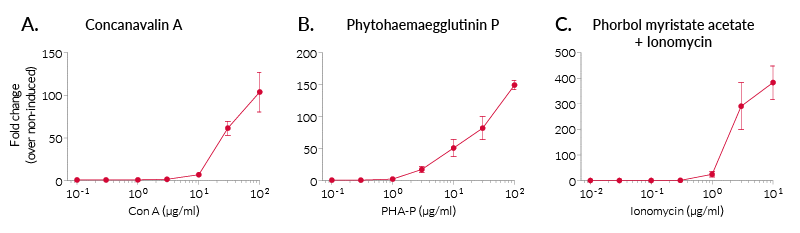
In Jurkat-Lucia™ NFAT cells, NFAT activation is achieved using cell-permeable small molecules triggering the calcium signaling (e.g. ionomycin) (see figures). Please note, that Jurkat-Lucia™ NFAT cells were engineered from a clone deficient in CD28 expression. In order to monitor the full T cell activation, InvivoGen also provides CD28-overexpressing Jurkat cells.
Jurkat-Lucia™ NFAT is the parental cell line for:
- Jurkat-Lucia™ NFAT-CD16 cells
- Jurkat-Lucia™ NFAT-CD16-Low cells
- Jurkat-Lucia™ NFAT-CD28 cells
- Jurkat-Lucia™ NFAT-CD32 cells
Key Features:
- Endogenous TCR, CD3, and NFAT expression
- Readily assessable NFAT activation by assessing the Lucia luciferase reporter activity
Applications:
- Screening of antibodies or small molecules mimicking the TCR stimulation and/or inducing the calcium signaling pathway
- Screening of NFAT targeting drug candidates
- Control cell line
1. Lee J-U., et al., 2018. Revisiting the Concept of Targeting NFAT to Control T Cell Immunity and Autoimmune Diseases. Front Immunol. DOI: 10.3389/fimmu.2018.02747.
2. Macian F., 2005. NFAT proteins: key regulators of T-cell development and function. Nat Rev Immunol. 5(6):472-484.
Specifications
Growth medium: IMDM, 2 mM L-glutamine, 25 mM HEPES, 10% (v/v) heat-inactivated fetal bovine serum (FBS), 100 U/ml penicillin, 100 µg/ml streptomycin, 100 µg/ml Normocin™
Antibiotic resistance: Zeocin®
Quality Control:
- Human CD3 expression has been verified by flow cytometry.
- The activation of NFAT has been confirmed following PMA/ionomycin and concanavalin A (ConA) treatment by measuring the levels of Lucia luciferase secreted.
- The stability for 20 passages following thawing has been verified.
- These cells are guaranteed mycoplasma-free.
Contents
- 3-7 x 106 Jurkat-Lucia™ NFAT cells in a cryovial or shipping flask
- 1 ml of Zeocin® (100 mg/ml)
- 1 ml of Normocin™ (50 mg/ml). Normocin™ is a formulation of three antibiotics active against mycoplasmas, bacteria, and fungi.
- 1 tube of QUANTI-Luc™ 4 Reagent, a Lucia luciferase detection reagent (sufficient to prepare 25 ml)
![]() Shipped on dry ice (Europe, USA, Canada, and some areas in Asia)
Shipped on dry ice (Europe, USA, Canada, and some areas in Asia)
FAQ Cell Lines
 Any questions about our cell lines ? Visit our frequently asked questions page
Any questions about our cell lines ? Visit our frequently asked questions page
Back to the top
Details
The current paradigm is that full activation of T cells requires at least two signals upon contact with APCs [1, 2]. Signal 1 is delivered through the interaction of the TCR and a specific antigenic peptide associated with an MHC (major histocompatibility complex) molecule on APCs. Signal 2 is delivered through the interaction of CD28, the prototypical T cell co-stimulatory molecule, and its ligands, CD80 or CD86, expressed by the APC.
Signal 1: TCR and [HLA::peptide]
The 'classical' and most represented TCR is an 80 to 90 kDa heterodimer composed of one α chain and one β chain. The αβTCR is a transmembrane protein expressed by developing and mature T cells. It features an extracellular ligand-binding pocket and a short cytoplasmic tail. Each αβTCR is restricted to a specific complex made of an antigenic peptide and a class I or class II MHC molecule. Human MHC molecules are also known as HLA (human leukocyte antigen). Because of its short cytoplasmic tail, the TCR, once engaged, lacks the ability to signal and requires non-covalent association with the CD3 to trigger downstream intracellular signaling and T cell activation [1, 2]. Importantly, signal 1 without co-stimulation results in T cell unresponsiveness or 'anergy', a tolerance mechanism that guards against premature activation.
Signal 2: CD28 and CD80/86
CD28 is a homodimeric and transmembrane protein expressed by T cells. Nearly all human CD4+ T cells and 50% of human CD8+ T cells express CD28. The CD28 interaction with CD80 (aka B7-1) or CD86 (aka B7-2) on APCs, in conjunction with TCR engagement, triggers a co-stimulation signal (signal 2). It results in T cell proliferation, cytokine production, cell survival and cellular metabolism [1, 2].
NFAT activation:
Most NFAT proteins are controlled by calcium influx upon TCR stimulation. Calcium binds calmodulin, which in turn activates calcineurin, a calmodulin-dependent phosphatase. Calcineurin dephosphorylates NFAT proteins, leading to their translocation into the nucleus, where they regulate the expression of many genes, either alone or in cooperation with other transcription factors [3, 4]. The co-engagement of CD28 triggers the activation of the AKT kinase, which contributes to enhancing NFAT translocation into the nucleus [4].
References:
1. Budd R.C. & Fortner K.A., 2017. Chapter 12 - T Lymphocytes. Kelley and Firestein's Textbook of Rheumatology (Tenth Edition). pages 189-206.
2. Smith-Garvin J.E. et al., 2009. T Cell Activation. Ann. Rev. Immunol. 27:591-619.
3. Lee J-U., et al., 2018. Revisiting the Concept of Targeting NFAT to Control T Cell Immunity and Autoimmune Diseases. Front Immunol. DOI: 10.3389/fimmu.2018.02747.
4. Macian F., 2005. NFAT proteins: key regulators of T-cell development and function. Nat Rev Immunol. 5(6):472-484.