NLRC4 KO RAW-ASC Cells
| Product | Unit size | Cat. code | Docs. | Qty. | Price | |
|---|---|---|---|---|---|---|
|
RAW-ASC KO-NLRC4 Cells NLRC4 Knockout & ASC expressing RAW 264.7 cells (murine macrophages) |
Show product |
3-7 x 10e6 cells |
raw-konlrc4
|
|
||
|
RAW-ASC KO-NLRC4 vial Additional cell vial |
Show product |
3-7 x 10e6 cells |
raw-konlrc4-av
|
Notification: Reference #raw-konlrc4-av can only be ordered together with reference #raw-konlrc4.
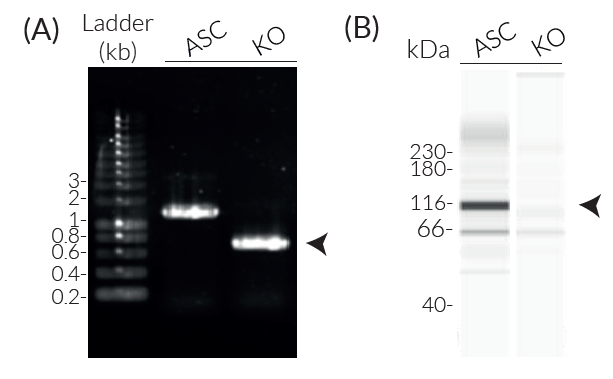
NLRC4 knockout in RAW 264.7 cells
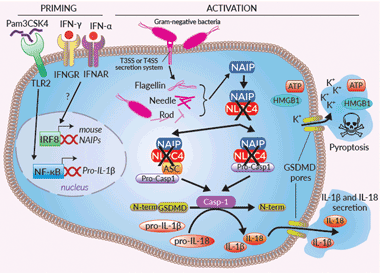
Inflammasome signaling in RAW-ASC KO-NLRC4 cells
NLRC4 (Nucleotide-binding domain (NBD) and leucin-rich repeat (LRR) receptor, CARD domain-containing protein 4, or IPAF) is a cytoplasmic protein which upon activation, triggers the assembly of an NLRC4 inflammasome [1-3].
InvivoGen has generated RAW-ASC KO-NLRC4 cells from the RAW-ASC cell line, which derives from the naturally ASC-deficient RAW 264.7 macrophage cell line [4]. RAW-ASC KO-NLRC4 cells stably express the transfected murine ASC gene and feature a stable knockout of the NLRC4 gene.
• RAW-ASC KO-NLRC4 cells – Knockout (KO) of the NLRC4 gene and expression of the murine ASC gene
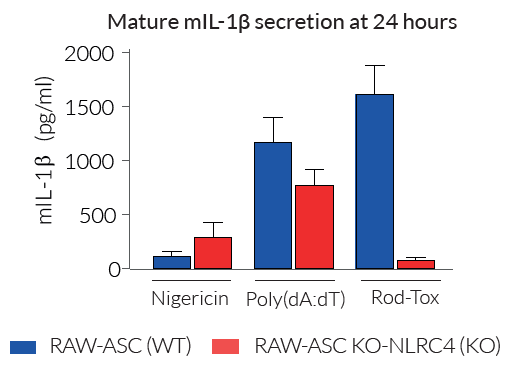
These cells exhibit impaired IL-1β secretion and pyroptosis upon incubation with known inducers of the NLRC4 inflammasome. Importantly, IL-1β secretion and pyroptosis remain unaffected upon incubation with other inflammasome inducers such as Nigericin (NLRP3 inducer).
FEATURES OF RAW-ASC KO-NLRC4 CELLS:
- Absence of functional NLRC4 protein expression
- Verified biallelic knockout of the NLRC4 gene (DNA sequencing, PCR, and Western blot)
- Altered IL-1β secretion and pyroptosis early upon NLRC4 inflammasome activation
![]() Download our Practical guide on Inflammasomes
Download our Practical guide on Inflammasomes
References:
1. Zhang L. et al., 2015. Cryo-EM structure of the activated NAIP2-NLRC4 inflammasome reveals nucleated polymerization. Science. 350:404-409.
2. Zhao Y. et al., 2011. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature. 477: 596-600.
3. Bauer R. & Rauch I., 2011. The NAIP/NLRC4 inflammasome in infection and pathology. Mol. Aspects Med. 2020 Jun 1:100863.
4. Pelerin P. et al., 2008. P2X7 receptor differentially couples to distinct release pathways for IL-1β in mouse macrophage. J. Immunol. 180:7147.
Specifications
Antibiotic resistance: Blasticidin
Growth medium: DMEM, 4.5 g/l glucose, 4 mM L-glutamine, 10% heat-inactivated fetal bovine serum (FBS), 100 U/ml penicillin, 100 µg/ml streptomycin, 100 µg/ml Normocin™
Test medium: DMEM without phenol red, 4.5 g/l glucose, 4 mM L-glutamine, 10% heat-inactivated FBS, 100 U/ml penicillin, 100 µg/ml streptomycin.
Note: Phenol red causes high background signal in the LDH (lactate dehydrogenase) assay used to monitor inflammasome-induced cell death.
Quality Control:
- Biallelic murine NLRC4 knockout has been verified by Western blot (Wes™) and functional assays.
- The stability for 20 passages, following thawing, has been verified.
- These cells are guaranteed mycoplasma-free.
Contents
- 3-7 x 106 RAW-ASC KO-NLRC4 cells in a cryovial or shipping flask
- 1 ml of Normocin™ (50 mg/ml). Normocin™ is a formulation of three antibiotics active against mycoplasma, bacteria, and fungi.
- 1 ml of Blasticidin (10 mg/ml)
![]() Shipped on dry ice (Europe, USA, Canada and some areas in Asia)
Shipped on dry ice (Europe, USA, Canada and some areas in Asia)
Details
Inflammasomes are cytoplasmic multi-protein complexes, characterized by a primary sensor, that assemble in response to infections and cellular damage. NLRC4 is an indirect sensor that must associate with NAIP (NLR family apoptosis inhibitory protein) to induce the assembly of an NLRC4 inflammasome. A single NAIP operates upstream of NLRC4 in humans and recognizes intracellular Flagellin, Needle, or Rod from several Gram-negative bacterial strains [1]. These ligands are sensed by NAIP upon bacterial invasion, or cytosolic translocation through the bacterial type III or IV secretion systems (T3SS or T4SS). Once recruited by NAIP, NLRC4 triggers a homo-polymerization through NBD-NBD interactions, allowing a CARD clustering [1]. The NLCR4 polymer further associates to pro-caspase-1, either through direct CARD-CARD interaction or through the binding of the ASC (apoptosis-associated speck-like protein) adaptor [1]. Activation of caspase-1 induces the maturation of pro-IL-1β/pro-IL-18, cleavage of the pore-forming gasdermin D (GSDMD), secretion of IL-1β/-18, and pyroptosis [1-3]. The NLRC4 inflammasome appears to protect mucosal barriers such as the lung, stomach, and intestine from invading bacteria [2]. Gain-of-function mutations have been described in human NLRC4 and are associated with auto-inflammatory conditions [3].
1. Zhang L. et al., 2015. Cryo-EM structure of the activated NAIP2-NLRC4 inflammasome reveals nucleated polymerization. Science. 350:404-409.
2. Zhao Y. et al., 2011. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature. 477: 596-600.
3. Bauer R. & Rauch I., 2011. The NAIP/NLRC4 inflammasome in infection and pathology. Mol. Aspects Med. 2020 Jun 1:100863.