RAW-Lucia™ ISG-KO-IRF5 Cells
| Product | Unit size | Cat. code | Docs. | Qty. | Price | |
|---|---|---|---|---|---|---|
|
RAW-Lucia™ ISG-KO-IRF5 Cells Murine RAW 264.7 macrophages - IRF5 knockout and IRF-Lucia Reporter Cells |
Show product |
3-7 x 10e6 cells |
rawl-koirf5
|
|
||
|
RAW-Lucia™ ISG-KO-IRF5 vial Additional cell vial |
Show product |
3-7 x 10e6 cells |
rawl-koirf5-av
|
--- |
Notification: Reference #rawl-koirf5-av can only be ordered together with reference #rawl-koirf5.
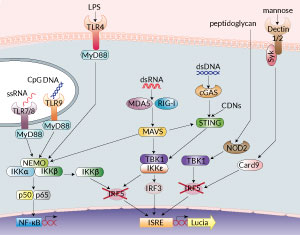
PRR signaling in RAW-Lucia™ ISG KO-IRF5 cells
IRF5 knockout reporter macrophages
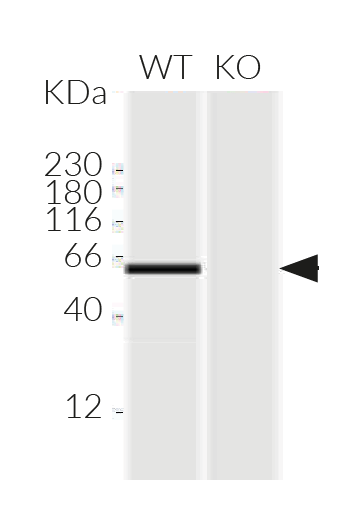
RAW-Lucia™ ISG-KO-IRF5 cells were generated from the RAW-Lucia™ ISG cell line through the stable knockout of the IRF5 gene.
Interferon regulatory receptor 5 (IRF5) is a transcription factor involved in the induction of type I interferon (IFN) downstream of pattern recognition receptors (PRRs), including the cytosolic nucleic acid sensors [1, 2]. There is increasing evidence that IRF5 plays a key role in numerous inflammatory and autoimmune diseases, including rheumatoid arthritis (RA) and inflammatory bowel disease [1, 3]. Importantly, because IRF5 acts in a cell-type and activity-specific manner, it is an attractive therapeutic target [1].
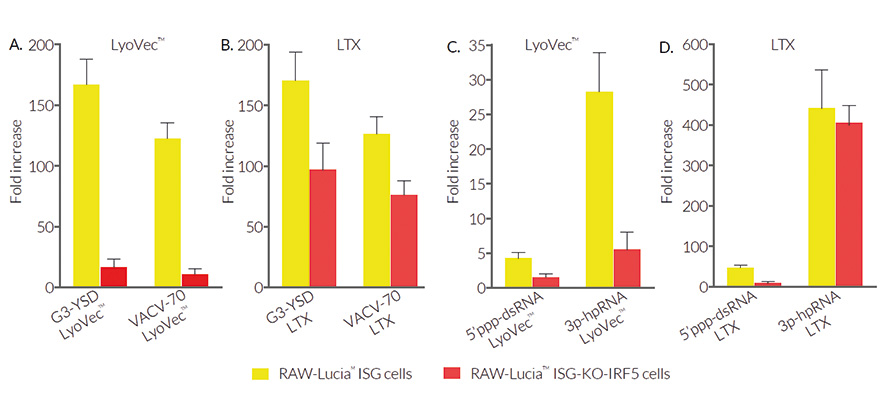
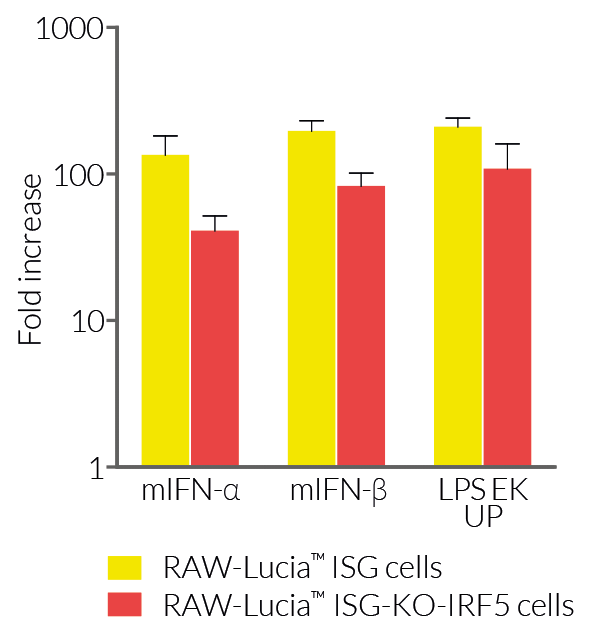
RAW-Lucia™ ISG-KO-IRF5 cells feature a Lucia luciferase reporter gene under the control of an ISG54 promoter enhanced by multimeric ISREs. Activation of the IRF pathway can be readily assessed by monitoring the activity of the secreted Lucia luciferase in the supernatant using the QUANTI-Luc™ 4 Lucia/Gaussia detection reagent. A differential response between RAW-Lucia™ ISG-KO-IRF5 cells and their parental cells is observed when using DNA- or RNA-based agonists with distinct transfection reagents (see Figures). RAW-Lucia™ ISG-KO-IRF5 cells retain the full ability to respond to type I interferons (IFN-α and IFN-β) and lipopolysaccharide (a TLR4 agonist) (see Figures).
Features:
- Verified knockout of the IRF5 gene (PCR, DNA sequencing, and Western blot)
- Functionally validated with a selection of PRR ligands and cytokines
- Readily assessable Lucia luciferase activity
Applications:
- Defining the role of IRF5 in PRR-induced signaling
- Highlighting possible overlapping PRR activation, or regulatory mechanisms
References:
1. Almuttaqi, H. & Udalova, I.A. 2019. Advances and challenges in targeting IRF5, a key regulator of inflammation. FEBS J 286, 1624-1637.
2. Zhao G-N. et al., 2015. Interferon regulatory factors: at the crossroads of immunity, metabolism, and disease. Biochim Biophys Acta. 2015 Feb;1852(2):365-78.
3. Kaur, A. et al. 2018. IRF5-mediated immune responses and its implications in immunological disorders. Int Rev Immunol 37, 229-248.
Specifications
Antibiotic resistance: Zeocin®
Growth medium: DMEM, 4.5 g/l glucose, 2 mM L-glutamine, 10% (v/v) heat-inactivated fetal bovine serum (FBS), 100 U/ml penicillin, 100 µg/ml streptomycin, 100 µg/ml Normocin™
Quality Control:
- Biallelic IRF5 knockout has been verified by PCR, DNA sequencing, Western blot, and functional assays.
- The stability for 20 passages, following thawing, has been verified.
- These cells are guaranteed mycoplasma-free.
Contents
- 3-7 x 106 of RAW-Lucia™ ISG-KO-IRF5 cells in a cryovial or shipping flask
- 1 ml of Normocin™ (50 mg/ml). Normocin™ is a formulation of three antibiotics active against mycoplasma, bacteria, and fungi.
- 1 ml of Zeocin® (100 mg/ml)
- 1 tube of QUANTI-Luc™ 4 Reagent, a Lucia luciferase detection reagent (sufficient to prepare 25 ml)
![]() Shipped on dry ice (Europe, USA, Canada, and some areas in Asia)
Shipped on dry ice (Europe, USA, Canada, and some areas in Asia)
Details
IRF5 background
Interferon regulatory factor 5 (IRF5) is a transcription factor that plays a central role in inflammation. It mediates the induction of type I interferons (IFNs) and proinflammatory cytokines through binding to ISRE and NF-κB motifs, respectively [1]. IRF5 has been implicated downstream of pattern recognition receptors (PRRs), including the cytosolic DNA/cyclic dinucleotide sensors cGAS/STING, the cytoplasmic RNA sensors, RIG-I and MDA5, Toll-like receptors (TLRs), the NOD-like receptor NOD2, and C-type lectin receptors such as Dectin 1 and Dectin 2 [1, 2]. Depending on the PRR that is triggered, IRF5 is activated through distinct mechanisms. TLR stimulation elicits IRF5 activation downstream of the MyD88 or the TRIF adaptors. Nucleic acid sensing by RIG-I, MDA5, cGAS or STING, elicits IRF5 activation through TBK1 (TANK-binding kinase 1). IRF5 can also be activated by the IKKβ kinase downstream of the MAVS adaptor associated with RIG-I or MDA5 [2]. IRF5 plays a key role in numerous inflammatory and autoimmune diseases including rheumatoid arthritis (RA) and inflammatory bowel disease [1, 3]. Importantly, because IRF5 acts in a cell-type and activity-specific manner, it is an attractive therapeutic target [1].
1. Almuttaqi, H. & Udalova, I.A. 2019. Advances and challenges in targeting IRF5, a key regulator of inflammation. FEBS J 286, 1624-1637.
2. Zhao, G.N. et al. 2015. Interferon regulatory factors: at the crossroads of immunity, metabolism, and disease. Biochim Biophys Acta. 2015 Feb;1852(2):365-78.
3. Kaur, A. et al. 2018. IRF5-mediated immune responses and its implications in immunological disorders. Int Rev Immunol 37, 229-248.