RAW-Lucia™ ISG-KO-IRF7 Cells
| Product | Unit size | Cat. code | Docs. | Qty. | Price | |
|---|---|---|---|---|---|---|
|
RAW-Lucia™ ISG-KO-IRF7 Cells Murine RAW 264.7 macrophages - IRF7 Knockout IRF-Lucia Luciferase Reporter Cells |
Show product |
3-7 x 10e6 cells |
rawl-koirf7
|
|
||
|
RAW-Lucia™ ISG-KO-IRF7 vial Additional cell vial |
Show product |
3-7 x 10e6 cells |
rawl-koirf7-av
|
Notification: Reference #rawl-koirf7-av can only be ordered together with reference #rawl-koirf7.
IRF7 knockout reporter mouse macrophages
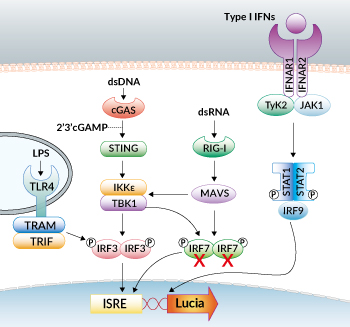
IRF signaling pathways in RAW-Lucia™ ISG-KO-IRF7 cells
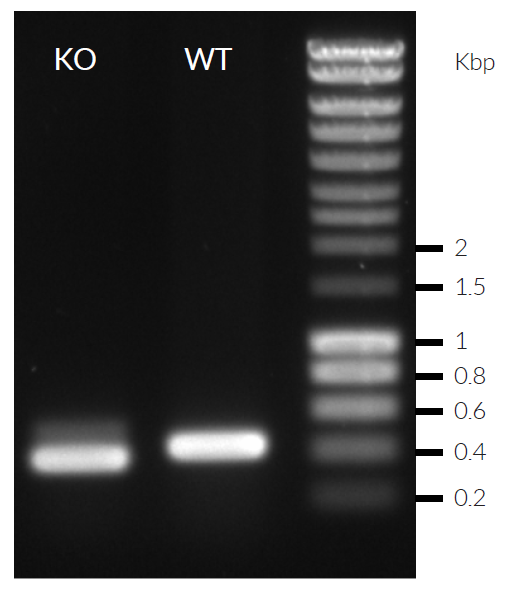
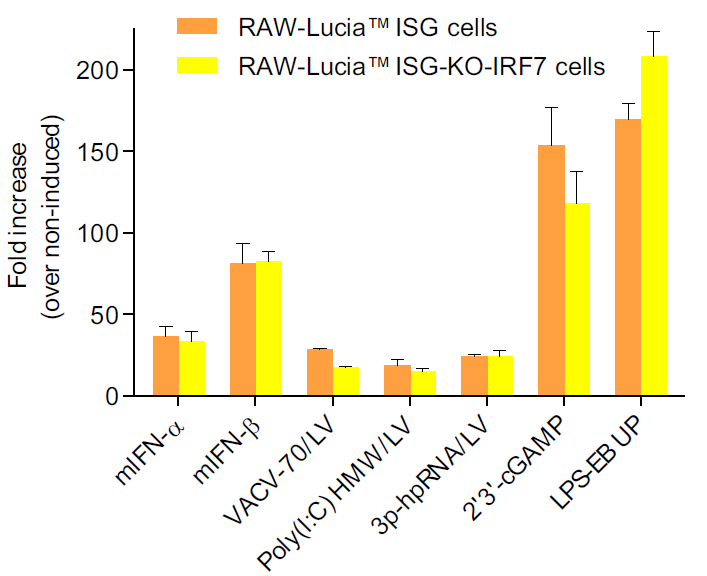
RAW-Lucia™ ISG-KO-IRF7 cells were generated from RAW-Lucia™ ISG cells through the stable gene knockout of the IRF7 gene. These cells feature an interferon regulatory factor (IRF)-inducible reporter gene. They derive from the murine RAW 264.7 macrophage cell line, which has been reported to express many pattern recognition receptors (PRRs) [1, 2]. RAW-Lucia™ ISG-KO-IRF7 and RAW-Lucia™ ISG cells can be used to study the role of IRF7 in a murine macrophage cell line by monitoring IRF-induced Lucia luciferase activity. Levels of secreted Lucia luciferase can be easily monitored using QUANTI-Luc™ 4 Lucia/Gaussia.
Interferon regulatory receptor 7 (IRF7) is a transcription factor involved in the activation of the type I interferon (IFN) response upon pathogenic infection [3-4]. IRF7 has been implicated downstream of several pattern recognition receptors (PRRs), including TLRs, RLRs, and DNA sensors [2]. IRF7 is highly homologous to IRF3, and they can form either homo- or heterodimers, which ultimately lead to IFN production [2].
Key Features:
- Verified knockout of the IRF7 gene (PCR and DNA sequencing)
- Functionally tested with a selection of PRR ligands and cytokines
- Readily assessable Lucia luciferase
Applications:
- Defining the role of IRF7 in PRR-induced signaling, or other cell signaling pathways
- Highlighting the possible functional overlap between IRF7 and other transcriptional factors
- Distinguishing the overlapping and differing roles of IRF7 and IRF3 in various signaling pathways
References
1. Lam E. et al., 2014. Adenovirus Detection by the cGAS/STING/TBK1 DNA Sensing Cascade. J Virol. 88:974-81.
2. Melchjorsen J. et al., 2005. Activation of innate defense against a paramyxovirus is mediated by RIG-I and TLR7 and TLR8 in a cell-type-specific manner. J Virol. 79:12944-51.
3. Ning, S. et al., 2011. IRF7: activation, regulation, modification and function. Genes Immun 12:399-414.
4. Perrotti, E. et al., 2013.IRF-7: an antiviral factor and beyond. Future Virol. 8(10):1007–20.
Specifications
Growth Medium: DMEM, 4.5 g/l glucose, 2 mM L-glutamine, 10% (v/v) heat-inactivated fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin, 100 μg/ml Normocin™
Antibiotic resistance: Zeocin®
Quality Control:
- Biallelic IRF7 knockout has been verified by PCR and DNA sequencing.
- The stability for 20 passages, following thawing, has been verified.
- These cells are guaranteed mycoplasma-free.
Contents
- 3-7 x 106 RAW-Lucia™ ISG-KO-IRF7 cells in a cryovial or shipping flask
- 1 ml of Normocin™ (50 mg/ml). Normocin™ is a formulation of three antibiotics active against mycoplasmas, bacteria, and fungi.
- 1 ml of Zeocin® (100 mg/ml)
- 1 tube of QUANTI-Luc™ 4 Reagent, a Lucia luciferase detection reagent (sufficient to prepare 25 ml)
![]() Shipped on dry ice (Europe, USA, Canada and some areas in Asia)
Shipped on dry ice (Europe, USA, Canada and some areas in Asia)
Details
IRF7 background
Interferon regulatory factor 7 (IRF7) is a transcription factor involved in the activation of the type I interferon (IFN) response upon pathogenic infection [1, 2]. IRF7 is constitutively expressed by plasmacytoid dendritic cells (pDCs), which are known as ‘professional’ type I IFN producing cells [1]. However, in all other cell types IRF7, unlike its closest homolog IRF3, is only expressed upon pathogenic infection [1, 2]. IRF7 has been implicated downstream of several pattern recognition receptors (PRRs), such as TLRs, RLRs, and DNA sensors [2]. Upon PRR stimulation, IRF7 is phosphorylated through the action of the IKK-related kinases TBK1 and IKKε, and forms a homo- or heterodimer with IRF3, ultimately leading to IFN production [2]. Despite both IRF3 and IRF7 being required for IFN production in most immune cells, they have been shown to also have distinct and unique features. IRF7 is solely responsible for the production of IFN-α [1]. Importantly, IRF7 is part of a positive feedback regulatory loop essential for sustained IFN responses and full protective adaptive immunity [2]. Furthermore, it has been established that IRF7 is involved in other cellular functions, including the regulation of oncogenesis [1, 2].
1. Ning, S. et al. 2011. IRF7: activation, regulation, modification and function. Genes Immun 12:399-414.
2. Perrotti, E. et al. 2013. IRF-7: an antiviral factor and beyond. Future Virol. 8(10):1007–20.