THP1-Blue™ KI-IP10 Cells
| Product | Unit size | Cat. code | Docs. | Qty. | Price | |
|---|---|---|---|---|---|---|
|
THP1-Blue™ KI-IP10 Cells CXCL10 Knockin Lucia luciferase & NF-κB SEAP Reporter Monocytes |
Show product |
3-7 x 10e6 cells |
thpb-ip10kilc
|
|
||
|
THP1-Blue™ KI-IP10 vial Additional cell vial |
Show product |
3-7 x 10e6 cells |
thpb-ip10kilc-av
|
Notification: Reference #thpb-ip10kilc-av can only be ordered together with reference #thpb-ip10kilc.
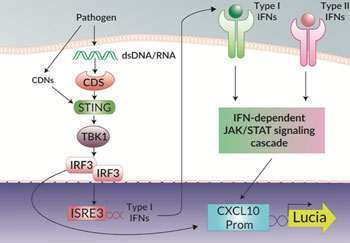
Examining the endogenous expression of CXCL10 (IP10)
THP1-Blue™ KI-IP10 cells have been designed to specifically examine the induction of the interferon-stimulated gene (ISG) IP10, also known as CXCL10, in response to bacterial and viral infection. Canonically, CXCL10 is known to be induced by the JAK/STAT signaling cascades activated by type I and II IFNs [1]. However, CXCL10 is also strongly induced by the activation of the STING/TBK1/IRF3 signaling axis by pathogen-derived nucleic acids and cyclic dinucleotides (CDNs) [2].
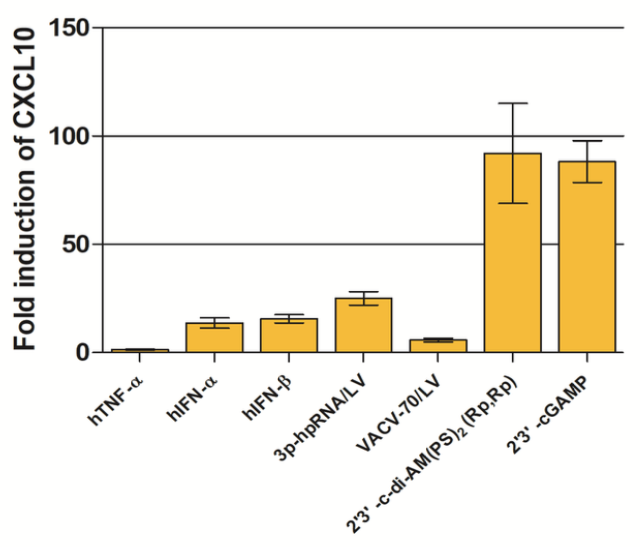
In THP1-Blue™ KI-IP10 cells the complete open reading frame of CXCL10 has been replaced with the Lucia luciferase reporter gene under the control of the endogenous promoter and thus allows the direct study of CXCL10 induction. Additionally, THP1-Blue™ KI-IP10 cells can be differentiated into macrophages, and CXCL10 expression, a classical marker of pro-inflammatory M1 macrophages, can be measured in response to stimulation. The induction of CXCL10 expression can be easily quantified by assessing the expression of the Lucia luciferase using QUANTI-Luc™ 4 Lucia/Gaussia, a Lucia and Gaussia luciferase detection reagent.
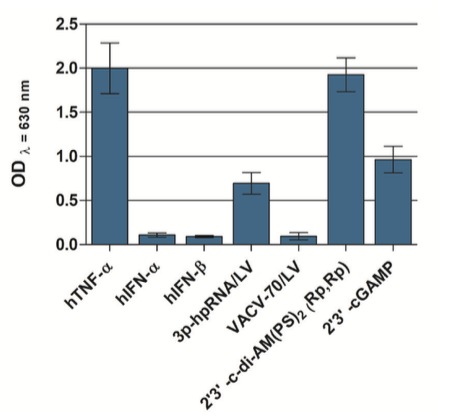
THP1-Blue™ KI-IP10 cells also stably express a secreted embryonic alkaline phosphatase (SEAP) reporter gene in response to the activation of the NF-κB pathway. Therefore, any crosstalk between the CXCL10 induction and NF-κB pathways can be studied [3]. Additionally, the independent activation of the NF-κB pathway, using an agonist such as TNF-α, is convenient for cross-sample normalization. The activation of the NF-κB pathway can be easily quantified by monitoring the activity of SEAP using QUANTI-Blue™ Solution, a SEAP detection reagent.
THP1-Blue™ KI-CXCL10 cells are resistant to blasticidin.
References:
1. Muller, M. et al., 2010. The chemokine receptor CXCR3 and its ligands CXCL9, CXCL10 and CXCL11 in neuroimmunity- a tale of conflict and conundrum. Neuropathol Appl Neurobiol. 36:368-387.
2. Motani, K. et al., 2015. DNA-Mediated Cyclic GMP-AMP Synthase-Dependent and -Independent Regulation of Innate Immune Responses. J Immunol. 194:4914‑4923.
3. Qi, X.F. et al., 2009. Essential involvement of cross-talk between IFN-gamma and TNF-alpha in CXCL10 production in human THP-1 monocytes. J Cell Physiol. 220(3):690-697.
Specifications
Antibiotic resistance: Blasticidin
Growth medium: RPMI 1640, 10% (v/v) heat-inactivated fetal bovine serum (HI-FBS; 30 min at 56°C), 100 μg/ml Normocin®, Pen-Strep (100 U/ml-100 μg/ml)
Quality control:
- Lucia luciferase gene knockin for IP10 has been verified by PCR and DNA sequencing.
- THP1-Blue™ KI-IP10 cells have been stimulated with and respond to type I IFNs as well as various agonists of STING, Toll-like receptors (TLR) and Rig-I-like receptors (RLRs).
- The stability for 20 passages following thawing has been verified.
- THP1-Blue™ KI-IP10 cells are guaranteed mycoplasma-free.
Contents
- 1 vial of THP1-Blue™ KI-IP10 cells (3-7 x 106 cells) in freezing medium
- 1 ml of Normocin® (50 mg/ml)
- 1 ml of Blasticidin (10 mg/ml)
- 1 tube of QUANTI-Luc™ 4 Reagent, a Lucia luciferase detection reagent (sufficient to prepare 25 ml)
- 1 ml of QB reagent and 1 ml of QB buffer (sufficient to prepare 100 ml of QUANTI-Blue™ Solution, a SEAP detection reagent)
![]() Shipped on dry ice (Europe, USA, Canada and some areas in Asia)
Shipped on dry ice (Europe, USA, Canada and some areas in Asia)