THP1-Dual™ KI-mSTING Cells
| Product | Unit size | Cat. code | Docs. | Qty. | Price | |
|---|---|---|---|---|---|---|
|
THP1-Dual™ KI-mSTING Cells Murine STING knockin - THP-1 reporter monocytes |
Show product |
3-7 x 10e6 cells |
thpd-mstg
|
|
||
|
THP1-Dual™ KI-mSTING vial Additional cell vial |
Show product |
3-7 x 10e6 cells |
thpd-mstg-av
|
Notification:
Reference #thpd-mstg-av can only be ordered together with reference #thpd-mstg.
Murine STING knockin dual reporter monocytes
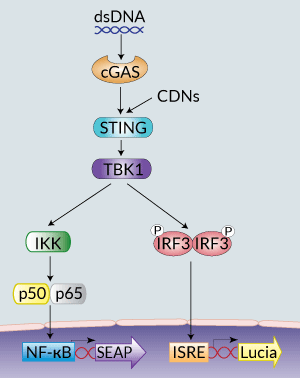
STING signaling in THP1-Dual™ KI-mSTING cells
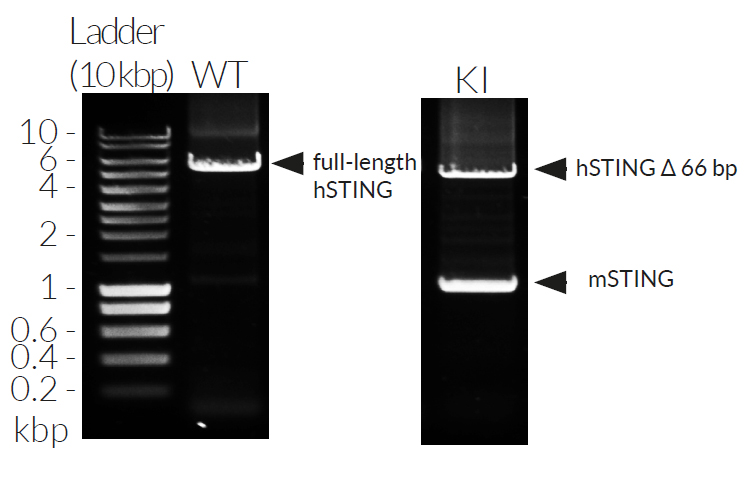
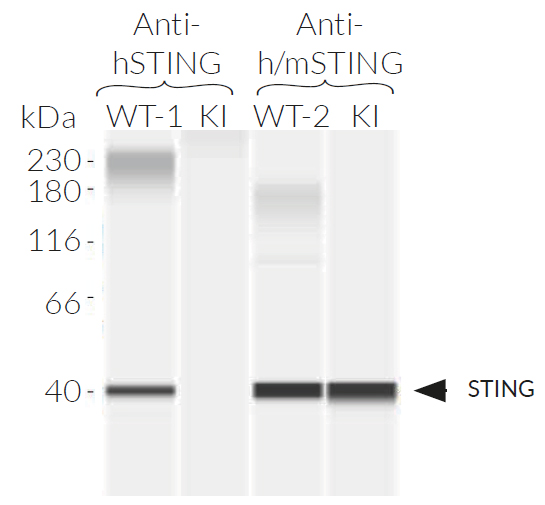
THP1-Dual™ KI-mSTING cells stably express the wild-type murine STING (stimulator of interferon genes, mSTING; also known as TMEM173) gene. They were generated by knockin (KI) of the mSTING intronless coding sequence (from ATG to TGA) into THP1-Dual™ KO-STING cells, which derive from the human THP-1 monocytes.
STING is a cytosolic pattern recognition receptor (PRR) that acts as a direct sensor of cyclic dinucleotides (CDNs) [1]. The binding of CDNs to STING leads to TANK-binding-kinase-I (TBK1)-mediated interferon regulatory factor (IRF3) activation and type I interferon (IFN) production [1] and to NF-κB-dependent inflammatory cytokine production [2].
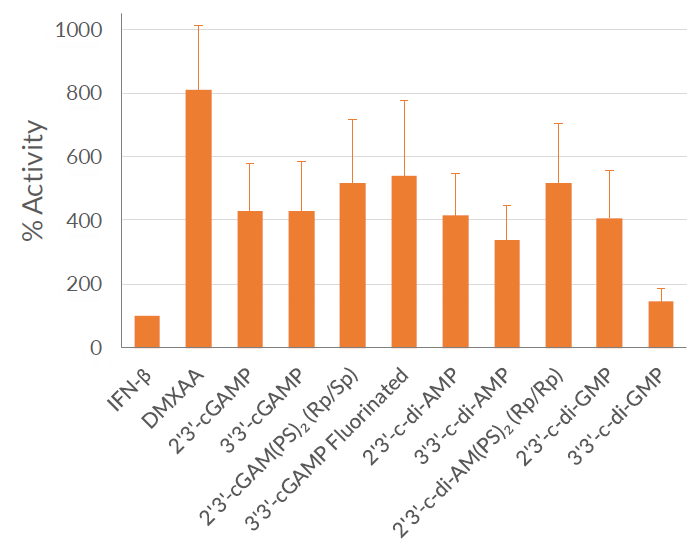
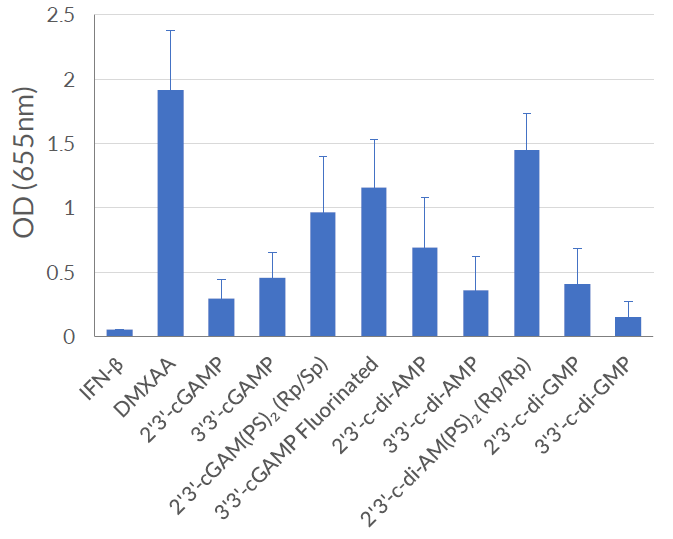
THP1-Dual™ KI-mSTING cells feature two reporter genes allowing the simultaneous study of the IRF pathway, by monitoring the activity of an inducible secreted Lucia luciferase, and the NF-κB pathway by monitoring the activity of an inducible SEAP (secreted embryonic alkaline phosphatase). Lucia luciferase and SEAP activities are readily assessable in the supernatant using QUANTI-Luc™ 4 Lucia/Gaussia and QUANTI-Blue™ Solution detection reagents, respectively.
As expected, THP1-Dual™ KI-mSTING cells exhibit a robust response to STING agonists such as 2’3’-cGAMP and 3’3’-cGAMP. In addition, these cells respond to DMXAA (5,6-Dimethylxanthenone 4-acetic acid), a potent activator of murine STING-dependent IFN responses, that does not activate human STING [3]. These cells are resistant to Blasticidin and Zeocin™.
Features of THP1-Dual™ KI-mSTING Cells:
- Knockin of the mSTING gene
- No functional hSTING expression
- IRF activation readily assessable using the Lucia luciferase reporter gene
- NF-κB activation readily assessable using the SEAP reporter gene
Validation of THP1-Dual™ KI-mSTING Cells:
- Murine STING knockin verified by PCR, DNA sequencing, and Western blot
- Functionally tested
References:
1. Sun L. et al., 2013. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. 339:786-91.
2. Ishikawa H. & Barber G.N. 2008. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature 455:674-8.
3. Zhang H. et al., 2015. Rat and human STINGs profile similarly towards anticancer/antiviral compounds. Sci Rep. 5:18035.
Specifications
Antibiotic resistance: Blasticidin and Zeocin™
Growth medium: RPMI 1640, 2 mM L-glutamine, 25 mM HEPES, 10% (v/v) fetal bovine serum (FBS), 100 U/ml penicillin, 100 µg/ml streptomycin, 100 µg/ml Normocin™
Quality Control:
- Murine STING knockin has been verified by PCR, DNA sequencing, western blot, and functional assays.
- The stability for 20 passages, following thawing, has been verified.
- These cells are guaranteed mycoplasma-free.
Contents
- 1 vial of THP1-Dual™ KI-mSTING cells (3-7 x 106 cells) in freezing medium
- 1 ml of Blasticidin (10 mg/ml)
- 1 ml of Zeocin™ (100 mg/ml)
- 1 ml of Normocin™ (50 mg/ml). Normocin™ is a formulation of three antibiotics active against mycoplasmas, bacteria, and fungi.
- 1 tube of QUANTI-Luc™ 4 Reagent, a Lucia luciferase detection reagent (sufficient to prepare 25 ml)
- 1 ml of QB reagent and 1 ml of QB buffer (sufficient to prepare 100 ml of QUANTI-Blue™ Solution, a SEAP detection reagent)
![]() Shipped on dry ice (Europe, USA, Canada, and some areas in Asia)
Shipped on dry ice (Europe, USA, Canada, and some areas in Asia)
Details
STING (stimulator of interferon genes; also known as TMEM173, MITA, MPYS, and ERIS) is essential for the interferon (IFN) response to microbial or self-DNA, and acts as a direct sensor of cyclic dinucleotides (CDNs). CDNs are important messengers in bacteria and innate immune agonists in mammals.
Upon bacterial infection, CDNs released into cells bind directly to STING leading to TANK-binding-kinase-I (TBK1)-mediated IFN regulatory factor (IRF3) activation and type I IFN production. Distinct variants of human STING (hSTING) that affect CDN recognition and signal transduction have been identified.