TLR4 KO Dual Reporter THP-1 Cells
| Product | Unit size | Cat. code | Docs. | Qty. | Price | |
|---|---|---|---|---|---|---|
|
THP1-Dual™ KO-TLR4 Cells TLR4 knockout NF-κB-SEAP and IRF-Lucia luciferase reporter monocytes |
Show product |
3-7 x 10e6 cells |
thpd-kotlr4
|
|
||
|
THP1-Dual™ KO-TLR4 vial Additional cell vial |
Show product |
3-7 x 10e6 cells |
thpd-kotlr4-av
|
Notification: Reference #thpd-kotlr4-av can only be ordered together with reference #thpd-kotlr4.
TLR4 knockout dual reporter monocytes
InvivoGen offers a new monocytic cell line to use in combination with its parental cell line THP1-Dual™ to monitor the TLR4 (Toll-like receptor 4)-dependent NF-kB and IRF responses induced by LPS (lipopolysaccharide):
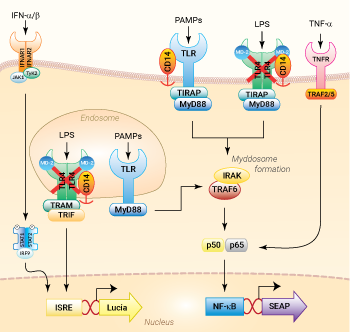
NF-κB and IRF signaling pathways in THP1-Dual™ KO-TLR4 cells
(click to enlarge and see legend)
— THP1-Dual™ KO-TLR4 cells
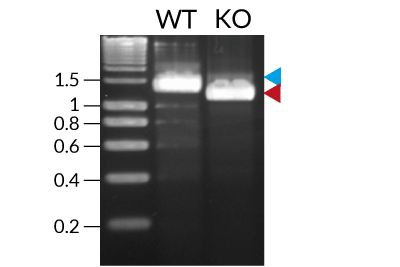
These cells were generated from the human THP1-Dual™ monocytic cell line through a biallelic knockout (KO) of the TLR4 gene. They also feature two inducible reporter genes, allowing the concomitant study of the NF-κB and IRF pathways, by monitoring the respective SEAP (secreted embryonic alkaline phosphatase) and Lucia luciferase activities in the cell supernatant.
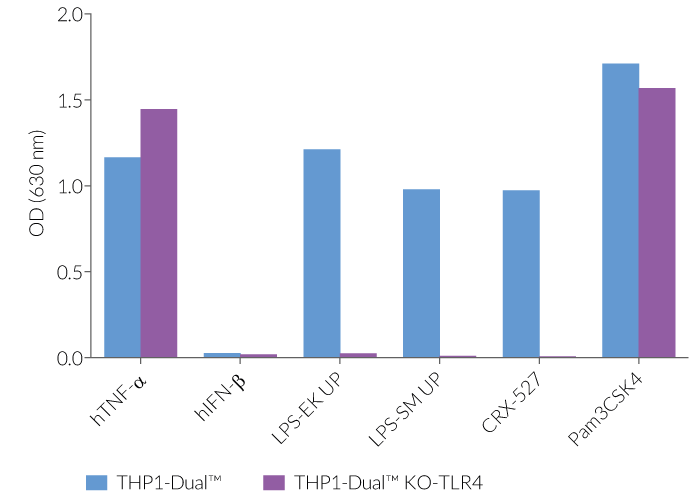
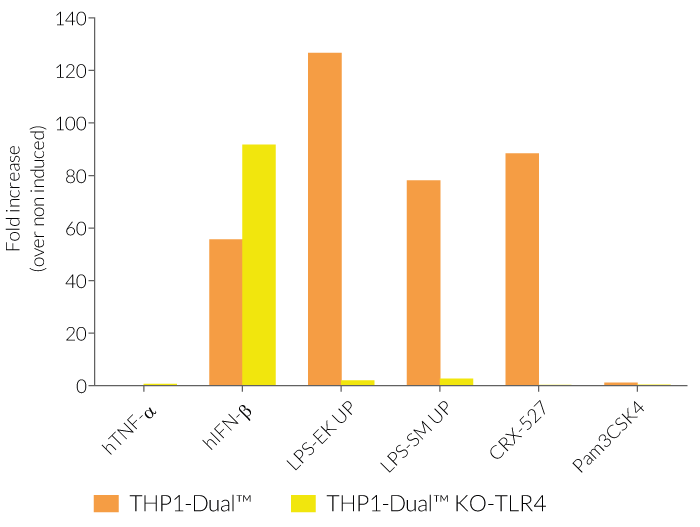
As expected, the NF-κB-mediated response is abolished in THP1-Dual™ KO-TLR4 cells upon incubation with LPS, when compared to their parental cell line (see figures). In THP1-Dual™-derived cells, TLR4-induced IRF-activation requires a differentiation step towards macrophages using PMA (Phorbol 12-myristate 13-acetate). In the TLR4-KO cells, this prior differentation treatment does not rescue the IRF-response upon stimulation with LPS due to the absence of the sensor (see figures).
Therefore, a loss in SEAP and/or Lucia expression in stimulated THP1-Dual™ KO-TLR4 cells compared to a strong signal in the THP1-Dual™ cells, suggests that the stimulus is either a TLR4-agonist or contaminated with LPS.
LPS is a major constituent of the outer membrane of Gram-negative bacteria, and elicits a potent innate immune response through the TLR4. The resulting signaling triggers the release of pro‑inflammatory cytokines like interleukin (IL)-1β and TNF-α [1]. Contamination by LPS is a major threat for health, research and industry, as it can interfere with signaling cascades or induce septic shock in vivo [2]. Thus, its accurate detection is crucial to obtain unbiased results and 'sterile' products.
Key Features:
- Verified knockout of the hTLR4 gene
- Functionally validated on a selection of TLR4 and other PRR ligands and cytokines
- Distinct monitoring of NF-κB and IRF activation by assessing the SEAP and Lucia luciferase activities
Applications:
- Determing the presence of LPS contamination in a sample
- Defining the role of TLR4 in PRR-induced signaling, or other cell signaling pathways
- Screening for TLR4 agonists or antagonists
- Highlighting possible overlap between TLR4 and other signaling pathways (e.g. TLR2)
References:
1. Cochet, F. et al. 2017. The Role of Carbohydrates in the Lipopolysaccharide (LPS)/Toll-Like Receptor 4 (TLR4) Signalling. Int J Mol Sci 18
2. Kuzmich, N.N. et al. 2017. TLR4 Signaling Pathway Modulators as Potential Therapeutics in Inflammation and Sepsis. Vaccines (Basel) 5.
Specifications
Growth medium: RPMI 1640, 2 mM L-glutamine, 25 mM HEPES, 10% (v/v) fetal bovine serum (FBS), 100 U/ml penicillin, 100 µg/ml streptomycin, 100 µg/ml Normocin™
Antibiotic resistance: Blasticidin and Zeocin®
Quality Control:
- TLR4 knockout has been verified by PCR, DNA sequencing, and functional assays.
- The stability for 20 passages, following thawing, has been verified.
- These cells are guaranteed mycoplasma-free.
Contents
- 3-7 x 106 THP1-Dual™ KO-TLR4 cells in a cryovial or shipping flask
- 1 ml of Normocin™ (50 mg/ml). Normocin™ is a formulation of three antibiotics active against mycoplasmas, bacteria, and fungi.
- 1 ml of Zeocin® (100 mg/ml)
- 1 ml of Blasticidin (10 mg/ml)
- 1 tube of QUANTI-Luc™ 4 Reagent, a Lucia luciferase detection reagent (sufficient to prepare 25 ml)
- 1 ml of QB reagent and 1 ml of QB buffer (sufficient to prepare 100 ml of QUANTI-Blue™ Solution, a SEAP detection reagent)
![]() Shipped on dry ice (Europe, USA, Canada, and some areas in Asia)
Shipped on dry ice (Europe, USA, Canada, and some areas in Asia)
Details
Toll-like receptor 4 signaling
The Toll-like receptor 4 (TLR4) was the first TLR identified and is an important pattern recognition receptor (PRR) in innate immunity and inflammation. It is found both on the cell surface and in endosomes of innate immune cells including monocytes and macrophages, as well as on intestinal epithelium and endothelial cells [1]. TLR4 can recognize pathogen- and damage-associated molecular patterns (PAMPs and DAMPs). However, it is primarily activated by lipopolysaccharide (LPS) and its toxic moiety Lipid A [2]. TLR4 does not directly interact with LPS but requires essential adaptor proteins [3]. The soluble LPS-binding protein (LBP) extracts monomeric LPS from the microbial membrane and transfers it to CD14 (cluster of differentiation 14). This membrane-bound protein then interacts with MD-2 (myeloid differentiation factor 2), constitutively associated with the TLR4 ectodomain. The ligand-loaded MD-2 subsequently binds to another TLR4/MD-2/LPS complex, leading to their dimerization [4]. Then, TLR4 triggers two distinct signaling cascades [5]:
- the MyD88-dependent activating NF-κB pathway (at the cell surface)
- the TRIF-dependent activating IRF pathway (in endosomes)
At the cell surface, activation of TLR4 initiates the TIRAP-MyD88-dependent pathway, ultimately leading to the activation of NF-κB and the production of a pro-inflammatory response. Also, the TLR4 complex can be endocytosed into endosomes in a CD14-mediated fashion. This results in the stimulation of IRF3 (interferon regulatory factor), which modulates the expression of type I IFN [3].
TLR4 signaling is crucial in both acute and chronic inflammatory disorders and thus, is an attractive target for novel treatments [1]. Stimulating drugs are useful for the development of vaccine adjuvants or cancer immunotherapeutics, whereas TLR4-inhibition is a therapeutic approach to treat septic shock or autoimmune inflammatory pathologies such as atherosclerosis [5].
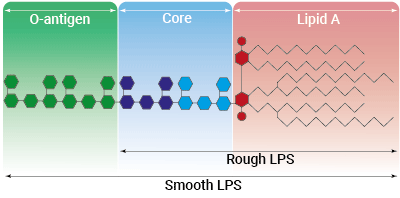
Rough vs smooth LPS
Lipopolysaccharide (LPS) is a major constituent of the outer membrane of Gram-negative bacteria. It comprises three covalently linked regions:
- the lipid A (endotoxin)
- the rough core oligosaccharide
- the O-antigenic side chain.
Wild-type LPS contains the O-side chain and is referred to as smooth (sLPS). Few bacterial strains (e.g. Salmonella Typhimurium, Brucella canis) lost the O-side chain. This mutated form is called rough (rLPS). Both sLPS and rLPS share the same receptor complex (TLR4-MD-2-CD14), but their mechanism of action differs. While CD14 is necessary for sLPS NF-κB and IRF signaling, it is dispensable for rLPS NF-κB signaling. It has been hypothesized that rLPS activates a broader range of cells (CD14 positive, low, and negative), accounting for higher toxicity [6].
Nevertheless, both LPS variations elicit potent innate immune responses. The resulting signaling triggers the release of pro‑inflammatory cytokines, which can lead to both acute and chronic inflammatory diseases. It is all about balance: small controlled amounts of LPS can be protective and large uncontrolled amounts can lead to disastrous outcomes, such as septic shock [7]. Despite its highly inflammatory nature, LPS has remarkable therapeutic potential and features many characteristics needed for an effective vaccine adjuvant [8].
References:
1. Ou, T. et al. 2018. The Pathologic Role of Toll-Like Receptor 4 in Prostate Cancer. Front Immunol 9, 1188.
2. Cochet, F. et al. 2017. The Role of Carbohydrates in the Lipopolysaccharide (LPS)/Toll-Like Receptor 4 (TLR4) Signalling. Int J Mol Sci 18
3. Kuzmich, N.N. et al. 2017. TLR4 Signaling Pathway Modulators as Potential Therapeutics in Inflammation and Sepsis. Vaccines (Basel) 5.
4.Tanimura N. et al. 2014. The attenuated inflammation of MPL is due to the lack of CD14-dependent tight dimerization of the TLR4/MD2 complex at the plasma membrane. Int Immunol.(6):307-14.
5. Romerio A, Peri F. 2020. Increasing the Chemical Variety of Small-Molecule-Based TLR4 Modulators: An Overview. Front Immunol.;11:1210.
6. Zanoni I,.et al., 2012. Similarities and differences of innate immune responses elicited by smooth and rough LPS. Immunol Lett. 2012 Feb 29;142(1-2):41-7.
7. Godowski, P., 2005. A smooth operator for LPS responses. Nat Immunol 6, 544–546.
8. McAleer, J.P. & Vella, A.T., 2010. Educating CD4 T cells with vaccine adjuvants: lessons from lipopolysaccharide. Trends Immunol 31, 429-435.