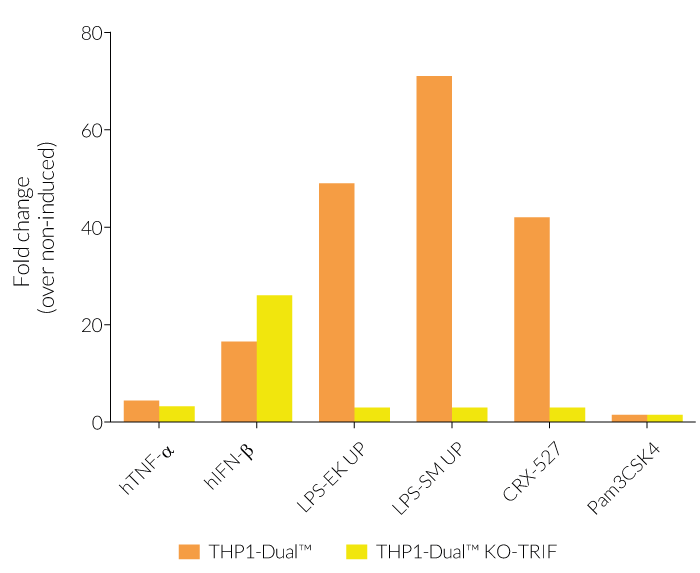TRIF KO Dual Reporter THP1 Cells
| Product | Unit size | Cat. code | Docs. | Qty. | Price | |
|---|---|---|---|---|---|---|
|
THP1-Dual™ KO-TRIF Cells TRIF knockout NF-κB-SEAP and IRF-Lucia luciferase reporter monocytes |
Show product |
3-7 x 10e6 cells |
thpd-kotrif
|
|
||
|
THP1-Dual™ KO-TRIF vial Additional cell vial |
Show product |
3-7 x 10e6 cells |
thpd-kotrif-av
|
Notification: Reference #thpd-kotrif-av can only be ordered together with reference #thpd-kotrif.
TRIF knockout dual reporter monocytes
InvivoGen offers a monocytic cell line specifically designed to monitor the TRIF-dependent NF-kB and IRF responses when used in combination with its parental cell line THP1-Dual™:
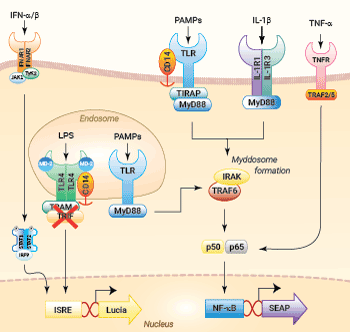
NF-κB and IRF signaling pathways in THP1-Dual™ KO-TRIF cells
(click to enlarge and see legend)
— THP1-Dual™ KO-TRIF cells
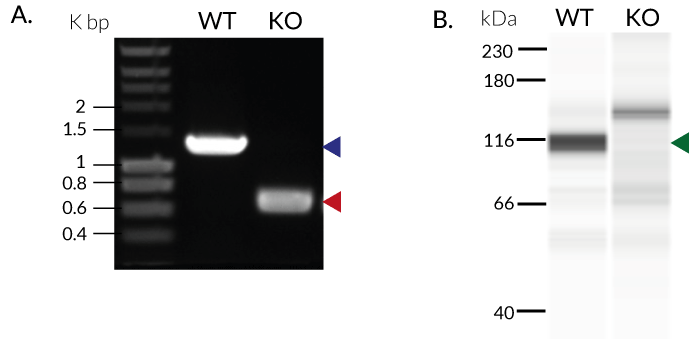
These cells were generated from the human THP1-Dual™ monocytic cell line through a biallelic knockout (KO) of the TRIF gene. They also feature two inducible reporter genes, allowing the concomitant study of the NF-κB and IRF pathways, by monitoring the respective SEAP (secreted embryonic alkaline phosphatase) and Lucia luciferase activities in the cell supernatant.
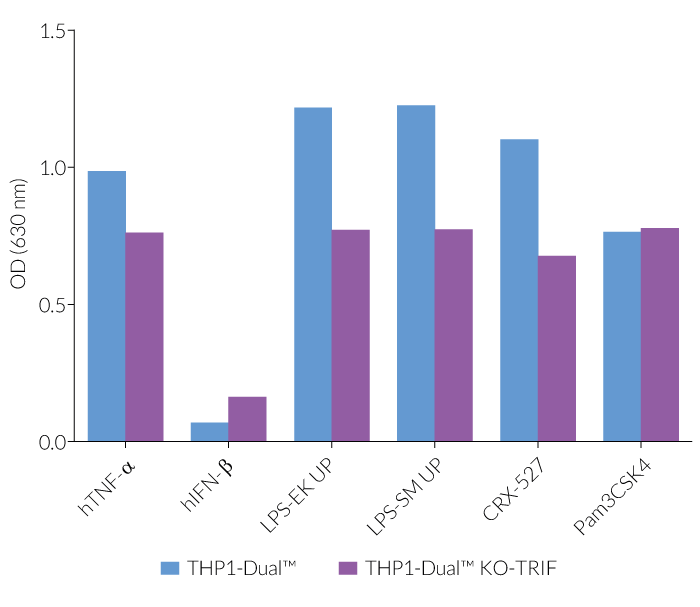
In THP1-Dual™-derived cells, the IRF-activation requires a differentiation step towards macrophages using PMA (Phorbol 12-myristate 13-acetate). As expected, this prior differentation treatment does not rescue the IRF-response in THP1-Dual™ KO-TRIF cells, when compared to their parental cell line (see figure). Since the MyD88-dependent NF-kB response is still functional in these cells, NF-kB can be still activated (see figure). The minor reduction of the NF-kB activation upon LPS stimulation in THP1-Dual™ KO-TRIF cells may be due to the involvement of TRIF in the late activation of NF-κB [1,2]. For comparing these two distinct TLR4 (Toll-like receptor) signaling cascades, MyD88 and TRIF, InvivoGen also offers THP1-Dual™ KO-MyD88 cells.
Please note: THP1‑Dual™ cells do not respond to TLR3-specific ligands.
Indeed, TIR-domain-containing adapter-inducing interferon-β (TRIF) is a key adaptor molecule in the TLR3- and TLR4-dependent signaling cascades. It is responsible for mediating TLR4-dependent/MyD88 independent signaling upon recognition of lipopolysaccharide (LPS) and TLR3-dependent signaling upon recognition of double-stranded (ds)RNA [3].
Key Features:
- Verified knockout of the TRIF gene
- Functionally validated on a selection of TLR4 and other PRR ligands and cytokines
- Distinct monitoring of NF-κB and IRF activation by assessing the SEAP and Lucia luciferase activities
Applications:
- Defining the role of TRIF in PRR-induced signaling (i.e. TLR3 & TLR4), or related cell signaling pathways
- Highlighting possible overlap between TRIF and other signaling pathways (e.g. MyD88)
References:
1. Najjar, M. et al. 2016. RIPK1 and RIPK3 Kinases Promote Cell-Death-Independent Inflammation by Toll-like Receptor 4. Immunity 45(1), 46-59.
2. Ciesielska, A. et al. 2020. TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell Mol Life Sci.
3. Ullah, M.O. et al. 2016. TRIF-dependent TLR signaling, its functions in host defense and inflammation, and its potential as a therapeutic target. J Leukoc Biol 100, 27-45.
Specifications
Growth medium: RPMI 1640, 2 mM L-glutamine, 25 mM HEPES, 10% (v/v) fetal bovine serum (FBS), 100 U/ml penicillin, 100 µg/ml streptomycin, 100 µg/ml Normocin™
Antibiotic resistance: Blasticidin and Zeocin®
Quality Control:
- Biallelic TRIF knockout has been verified by PCR, DNA sequencing, Western blot, and functional assays.
- The stability for 20 passages, following thawing, has been verified.
- These cells are guaranteed mycoplasma-free.
Contents
- 3-7 x 106 THP1-Dual™ KO-TRIF cells in a cryovial or shipping flask
- 1 ml of Normocin™ (50 mg/ml). Normocin™ is a formulation of three antibiotics active against mycoplasmas, bacteria, and fungi.
- 1 ml of Zeocin® (100 mg/ml)
- 1 ml of Blasticidin (10 mg/ml)
- 1 tube of QUANTI-Luc™ 4 Reagent, a Lucia luciferase detection reagent (sufficient to prepare 25 ml)
- 1 ml of QB reagent and 1 ml of QB buffer (sufficient to prepare 100 ml of QUANTI-Blue™ Solution, a SEAP detection reagent)
![]() Shipped on dry ice (Europe, USA, Canada, and some areas in Asia)
Shipped on dry ice (Europe, USA, Canada, and some areas in Asia)
FAQ Cell Lines
 Any questions about our cell lines ? Visit our frequently asked questions page
Any questions about our cell lines ? Visit our frequently asked questions page
Back to the top
Details
TRIF Background
TRIF (TIR-domain-containing adapter-inducing interferon-β), also known as TIR-containing adaptor molecule-1 or TICAM-1, is a key adaptor molecule in the TLR (Toll-like receptor)-dependent signaling cascades. It plays a central role in a range of innate immune responses including anti-viral host defenses, cell death, and homeostasis [1]. Specifically, TRIF is responsible for mediating TLR4-dependent/MyD88 independent signaling upon recognition of lipopolysaccharide (LPS). Additionally, it is involved in TLR3-dependent signaling upon recognition of double-stranded (ds)RNA [1]. Ultimately, TRIF recruitment activates interferon regulatory factor-3 (IRF3) which regulates both type I interferon (IFN) gene expression and a range of IFN-stimulated genes (ISG) [1].
Additionally, TRIF has been shown to induce TLR4-dependent late activation of NF-κB through recruitment and activation of TRAF6 or receptor-interacting serine/threonine protein kinases 1 and 3 (RIPK1 and RIPK3) [2,3]. In addition to the key role played by RIPK1 & RIPK3 in necroptosis, their activation in this context has been shown to lead to the acute expression of pro-inflammatory cytokines [2]. Importantly, TLR4-dependent TRIF activation is independent of its activation of the MyD88-dependent (at the cell surface) pathway, which is implicated in ‘early’ activation of NF-κB and a subsequent pro-inflammatory response [3].
Toll-like receptor 4 signaling
The Toll-like receptor 4 (TLR4) was the first TLR identified and is an important pattern recognition receptor (PRR) in innate immunity and inflammation. It is found both on the cell surface and in endosomes of innate immune cells including monocytes and macrophages, as well as on intestinal epithelium and endothelial cells [4]. TLR4 can recognize pathogen- and damage-associated molecular patterns (PAMPs and DAMPs). However, it is primarily activated by LPS and its toxic moiety Lipid A [6]. TLR4 does not directly interact with LPS, but requires essential adaptor proteins [5]. The soluble LPS-binding protein (LBP) extracts monomeric LPS from the microbial membrane and transfers it to CD14 (cluster of differentiation 14). This membrane-bound protein then interacts with MD-2 (myeloid differentiation factor 2), which is constitutively associated with the TLR4 ectodomain. The ligand-loaded MD-2 subsequently binds to another TLR4/MD-2/LPS complex, leading to their dimerization [7]. Interestingly, TLR4 is the only TLR able to trigger two distinct signaling cascades [4]:
- the MyD88-dependent activating NF-κB pathway (at the cell surface)
- the TRIF-dependent activating IRF pathway (in endosomes)
At the cell surface, activation of TLR4 initiates the TIRAP-MyD88-dependent pathway, ultimately leading to the activation of NF-κB and the production of a pro-inflammatory response. Also, the TLR4 complex can be endocytosed into endosomes in a CD14-mediated fashion. This results in the the stimulation of IRF3 (interferon regulatory factor), which modulates the expression of type I IFN [5].
TLR4 signaling is crucial in both acute and chronic inflammatory disorders and thus, is an attractive target for novel treatments [4]. Stimulating drugs are useful for the development of vaccine adjuvants or cancer immunotherapeutics, whereas TLR4-inhibition is a therapeutic approach to treat septic shock or autoimmune inflammatory pathologies such as atherosclerosis [8].
References:
1. Ullah, M.O. et al. 2016. TRIF-dependent TLR signaling, its functions in host defense and inflammation, and its potential as a therapeutic target. J Leukoc Biol 100, 27-45.
2. Najjar, M. et al. 2016. RIPK1 and RIPK3 Kinases Promote Cell-Death-Independent Inflammation by Toll-like Receptor 4. Immunity 45(1), 46-59.
3. Ciesielska, A. et al. 2020. TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell Mol Life Sci.
4. Ou, T. et al. 2018. The Pathologic Role of Toll-Like Receptor 4 in Prostate Cancer. Front Immunol 9, 1188.
5. Kuzmich, N.N. et al. 2017. TLR4 Signaling Pathway Modulators as Potential Therapeutics in Inflammation and Sepsis. Vaccines (Basel) 5.
6. Cochet, F. et al. 2017. The Role of Carbohydrates in the Lipopolysaccharide (LPS)/Toll-Like Receptor 4 (TLR4) Signalling. Int J Mol Sci 18
7.Tanimura N. et al. 2014. The attenuated inflammation of MPL is due to the lack of CD14-dependent tight dimerization of the TLR4/MD2 complex at the plasma membrane. Int Immunol.(6):307-14.
8. Romerio A, Peri F. 2020. Increasing the Chemical Variety of Small-Molecule-Based TLR4 Modulators: An Overview. Front Immunol.;11:1210.