THP1-Dual™ KO-MyD88 Cells
| Product | Unit size | Cat. code | Docs. | Qty. | Price | |
|---|---|---|---|---|---|---|
|
THP1-Dual KO-MyD Cells MyD88 knockout NF-κB-SEAP and IRF-Lucia luciferase reporter monocytes |
Show product |
3-7 x 10e6 cells |
thpd-komyd
|
|
||
|
THP1-Dual KO-MyD vial Additional cell vial |
Show product |
3-7 x 10e6 cells |
thpd-komyd-av
|
Notification: Reference #thpd-komyd-av can only be ordered together with reference #thpd-komyd.
MyD88 knockout dual reporter monocytes
InvivoGen offers a monocytic cell line specifically designed to monitor the MyD88-dependent NF-kB responses when used in combination with its parental cell line THP1-Dual™:
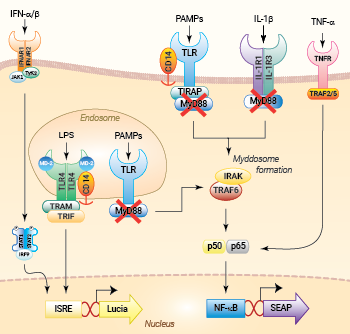
NF-κB and IRF signaling pathways in THP1-Dual™ KO-MyD cells
(click to enlarge and see legend)
— THP1-Dual™ KO-MyD cells
These cells were generated from the human THP1-Dual™ monocytic cell line through a biallelic knockout (KO) of the MyD88 gene. They also feature two inducible reporter genes, allowing the concomitant study of the NF-κB and IRF pathways, by monitoring the respective SEAP (secreted embryonic alkaline phosphatase) and Lucia luciferase activities in the cell supernatant.
THP1-Dual™ KO‑MyD cells are unable to respond to the activation of receptors whose signaling is dependent on MyD88, such as TLR2, TLR4, TLR5, TLR8 and IL-1Rs, when compared to their parental cell line THP1-Dual™. However, they remain responsive to MyD88‑independent receptors, such as TNFR (see figure). The activation of the IRF pathway is unaffected by the knockout of the MyD88 gene. For comparing the two distinct signaling cascades, MyD88 and TRIF, InvivoGen also offers THP1-Dual™ KO-TRIF cells.
MyD88, the 'Myeloid Differentiation Primary Response Gene 88' is a central node of inflammatory signaling pathways downstream of members of the Toll-like receptor (TLR) and interleukin-1 receptor (IL-1R) families [1]. Based on its biological role, MyD88 has been in the spotlight as a therapeutic target for different inflammatory disorders [1-2].
Key Features:
- Verified knockout of the MyD88 gene
- Functionally validated on a selection of TLR ligands and cytokines
- Distinct monitoring of NF-κB and IRF activation by assessing the SEAP and Lucia luciferase activities
Applications:
- Defining the role of MyD88 in PRR-induced signaling (e.g. TLR2, TLR4), or related cell signaling pathways
- Highlighting possible overlap between MyD88 and other signaling pathways
References:
1. Deguine J, Barton GM. 2014. MyD88: a central player in innate immune signaling. F1000Prime Rep.;6:97.
2. Chen L, et al., 2020. Myeloid Differentiation Primary Response Protein 88 (MyD88): The Central Hub of TLR/IL-1R Signaling. J Med Chem. ;63(22):13316-13329.
Specifications
Growth medium: RPMI 1640, 2 mM L-glutamine, 25 mM HEPES, 10% heat-inactivated fetal bovine serum, 100 μg/ml Normocin™, Pen-Strep (100 U/ml-100 μg/ml)
Antibiotic resistance: Blasticidin and Zeocin®
Quality control:
- Biallelic MyD88 knockout has been verified by PCR, DNA sequencing, Western blot, and functional assays.
- The stability for 20 passages, following thawing, has been verified.
- These cells are guaranteed mycoplasma-free.
Contents
- 3-7 x 106 THP1-Dual™ KO-MyD cells in a cryovial or shipping flask
- 1 ml of Normocin™ (50 mg/ml). Normocin™ is a formulation of three antibiotics active against mycoplasmas, bacteria and fungi.
- 1 ml of Zeocin® (100 mg/ml)
- 1 ml of Blasticidin (10 mg/ml)
- 1 tube of QUANTI-Luc™ 4 Reagent, a Lucia luciferase detection reagent (sufficient to prepare 25 ml)
- 1 ml of QB reagent and 1 ml of QB buffer (sufficient to prepare 100 ml of QUANTI-Blue™ Solution, a SEAP detection reagent)
![]() Shipped on dry ice (Europe, USA, Canada, and some areas in Asia)
Shipped on dry ice (Europe, USA, Canada, and some areas in Asia)
FAQ Cell Lines
 Any questions about our cell lines ? Visit our frequently asked questions page
Any questions about our cell lines ? Visit our frequently asked questions page
Back to the top
Details
MyD88
The Myeloid Differentiation Primary Response Gene 88 (MyD88) is the canonical adaptor for inflammatory signaling pathways downstream of members of the Toll-like receptor (TLR) and interleukin-1 receptor (IL-1R) families [1]. Based on the adaptor proteins involved, the TLR/IL-1Rs pathways can be classified into MyD88-dependent and MyD88-independent (TRIF-dependent) responses. With the exception of TLR3 and TLR4, all TLRs mediate the downstream signaling pathway via MyD88 [2-3].
The MYD88 protein contains three key domains: a N-terminal death domain (DD), a short intermediate domain (INT), and a C-terminal Toll-IL1R domain (TIR). In the resting state, inactive MyD88 appears to be dispersed in the cytosol [3]. Stimulation of TLRs and IL1R leads to the direct recruitment of cytosolic MyD88. In the case of TLR2 and TLR4 signaling, a bridging adaptor, TIRAP (aka Mal), is required for MyD88 recruitment. Upon receptor-adaptor binding through TIR-TIR interactions, an oligomeric protein complex called 'the Myddosome' is assembled [3].
The Myddosome
Within this complex, activated MyD88 recruits IL-1R associated kinase 4 (IRAK4), a serine-threonine kinase, via their DD domains. Together they phosphorylate IRAK1 or IRAK2. Phosphorylated IRAK1 and IRAK2 interact with tumor necrosis factor receptor-associated factor 6 (TRAF6), resulting in activation of transforming growth factor beta-activated kinase 1 (TAK1). TAK1 subsequently activates the inhibitor of the NF-κB kinase (IKK) complex. After activation, this complex phosphorylates the inhibitor of NF-κB (IκB) proteins that are bound to NF-κB, which prevent the translocation of NF-κB to the nucleus. Phosphorylation of these IκB proteins results in ubiquitylation and proteasomal degradation of IκB and release of the NF-κB subunits. Subsequently, NF-κB migrates to the nucleus where it bind to specific DNA-binding sites and induces gene expression [4].
Pathology
Based on its biological role, MyD88 has been in the spotlight as a therapeutic target for different inflammatory disorders. Gain-of-function mutations or overexpression of MyD88 persistently activate NF-κB, resulting in inflammatory response syndrome, cancer, and autoimmune diseases [3]. Sequencing studies recently identified mutations in the MYD88 gene as an important oncogenic driver and biomarker in B-cell lymphomas [4]. Indeed, MyD88 deficiency and loss-of-function has anti-inflammatory effects in several conditions such as arthritis, artherosclerosis, and autoimmune CNS diseases. Also, it alters MyD88-independent pathways [3]. Therefore, understanding the diverse functions of this unique adaptor protein and finding inhibitors targeting MyD88 will offer therapeutic opportunities for numerous indications [3].
References:
1. Deguine J, Barton GM. 2014. MyD88: a central player in innate immune signaling. F1000Prime Rep.;6:97.
2. Chao Zheng et al., 2020. Inflammatory Role of TLR-MyD88 Signaling in Multiple Sclerosis. Front. Mol. Neurosci. 12:314.
3. Chen L, et al., 2020. Myeloid Differentiation Primary Response Protein 88 (MyD88): The Central Hub of TLR/IL-1R Signaling. J Med Chem. ;63(22):13316-13329.
4. de Groen RAL, et al., 2019. MYD88 in the driver's seat of B-cell lymphomagenesis: from molecular mechanisms to clinical implications. Haematologica. 2019 Dec;104(12):2337-2348.








