IRF3 KO Dual Reporter THP1 Cells
| Product | Unit size | Cat. code | Docs. | Qty. | Price | |
|---|---|---|---|---|---|---|
|
THP1-Dual™ KO-IRF3 Cells IRF3 knockout NF-κB-SEAP and IRF-Lucia Reporter Cells |
Show product |
3-7 x 10e6 cells |
thpd-koirf3
|
|
||
|
THP1-Dual™ KO-IRF3 vial Additional cell vial |
Show product |
3-7 x 10e6 cells |
thpd-koirf3-av
|
Notification: Reference #thpd-koirf3-av can only be ordered together with reference #thpd-koirf3.
IRF3 knockout dual reporter monocytes
Nucleic acid sensing signaling in THP1-Dual™ KO-IRF3 cells
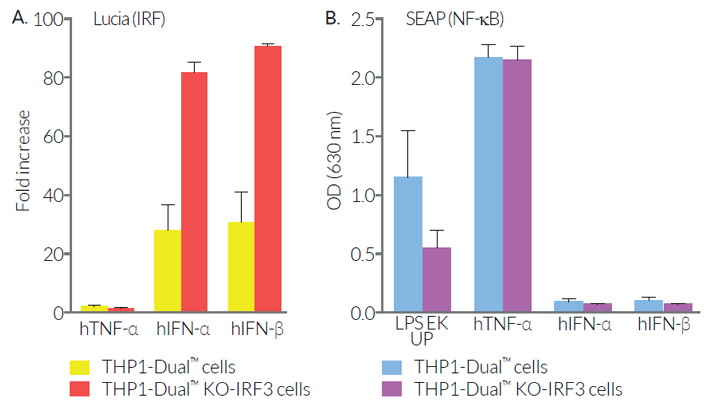
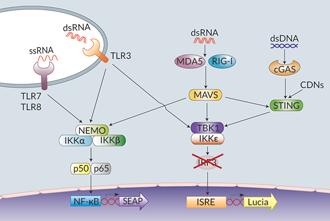
THP1-Dual™ KO-IRF3 cells were generated from the THP1-Dual™ cell line, which is derived from the human THP-1 monocytes, through the stable knockout of the IRF3 gene. IRF3 (interferon regulatory factor 3) is a critical player in the innate immune responses to virus infections. Viral nucleic acids in the cytosol are recognized by pattern recognition receptors (PRRs) such as Toll-like receptors (TLRs), RIG-I-like receptors (RLRs), or cytosolic DNA sensors (e.g. the cyclic GMP-AMP synthase cGAS). Upon ligand binding, these receptors trigger the production of type I interferons (IFNs) through the activation of the TBK1/IKKε-IRF3/IRF7 pathway [1]. Activated PRRs also trigger the production of pro-inflammatory cytokines through activation of the NF-κB pathway [1].
THP1-Dual™ KO-IRF3 and THP1-Dual™ cells feature two reporter genes allowing the simultaneous study of the IRF pathway, by monitoring the activity of an inducible secreted Lucia luciferase, and the NF-κB pathway by monitoring the activity of an inducible SEAP (secreted embryonic alkaline phosphatase). Lucia luciferase and SEAP activities are readily assessable in the supernatant using QUANTI-Luc™ 4 Lucia/Gaussia and QUANTI-Blue™ Solution detection reagents, respectively.
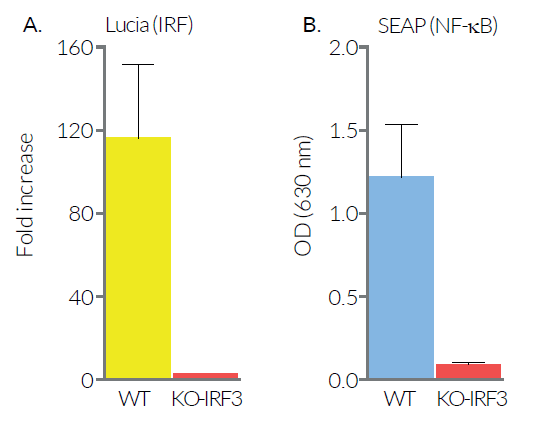
As expected, the responses of THP1-Dual™ KO-IRF3 cells to the cyclic dinucleotide 2'3'-cGAMP (a STING agonist) and to lipopolysaccharide (a TLR4 agonist) are impaired. However, differential responses (either no effect, decrease or increase) are observed when using RNA or DNA agonists coupled with transfection reagents. THP1-Dual™ KO-IRF3 cells retain the ability to respond to cytokines such as type I IFNs and TNF-α. These cells are resistant to Blasticidin and Zeocin™.
Features of THP1-Dual™ KO-IRF3 cells:
- Biallelic knockout of the IRF3 gene
- Readily assessable Lucia luciferase and SEAP reporter activity
Applications for THP1-Dual™ KO-IRF3 cells:
- Defining the role of IRF3 in PRR-induced signaling
- Highlighting possible overlapping PRR activation or regulatory mechanisms (see 'Details' tab)
Validation of THP1-Dual™ KO-IRF3 cells:
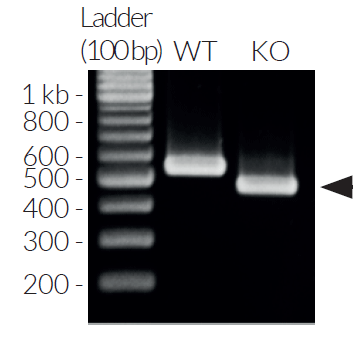
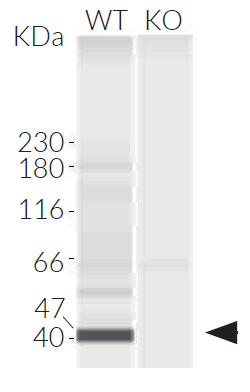
- IRF3 knockout verified by PCR, DNA sequencing, and western-blot
- Functionally tested
References:
1. Iurescia S. et al., 2018. Nucleic acid sensing machinery: targeting innate immune system for cancer therapy. Recent Pat. Anticancer Drug Discov. 13: 2-17
Back to the topSpecifications
Antibiotic resistance: Blasticidin and Zeocin™
Growth medium: RPMI 1640, 2 mM L-glutamine, 25 mM HEPES, 10% (v/v) fetal bovine serum (FBS), 100 U/ml penicillin, 100 µg/ml streptomycin, 100 µg/ml Normocin™
Quality Control:
- Biallelic IRF3 knockout has been verified by PCR, DNA sequencing, western blot, and functional assays.
- The stability for 20 passages, following thawing, has been verified.
- These cells are guaranteed mycoplasma-free.
Contents
- 1 vial of THP1-Dual™ KO-IRF3 cells (3-7 x 106 cells) in freezing medium
- 1 ml of Normocin™ (50 mg/ml). Normocin™ is a formulation of three antibiotics active against mycoplasmas, bacteria, and fungi.
- 1 ml of Zeocin™ (100 mg/ml)
- 1 ml of Blasticidin (10 mg/ml)
- 1 tube of QUANTI-Luc™ 4 Reagent, a Lucia luciferase detection reagent (sufficient to prepare 25 ml)
- 1 ml of QB reagent and 1 ml of QB buffer (sufficient to prepare 100 ml of QUANTI-Blue™ Solution, a SEAP detection reagent)
![]() Shipped on dry ice (Europe, USA, Canada, and some areas in Asia)
Shipped on dry ice (Europe, USA, Canada, and some areas in Asia)
Details
THP1-Dual™ KO-IRF3 cells have been functionally tested using various sources of nucleic acids (see the validation data document).
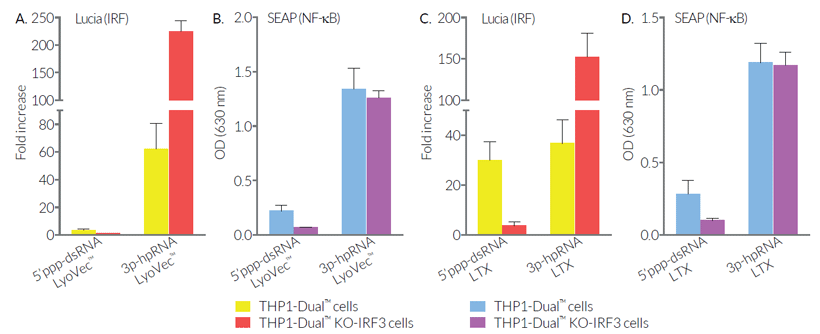
As expected, IRF and NF-κB responses are severely impaired when the THP1-Dual™ KO-IRF3 cells are incubated with STING agonists, such as 2’3’-cGAMP, which do not require transfection to access the cytosol. However, differential responses are observed when using RNA agonists with transfection reagents. The weak agonist 5’ppp-dsRNA loses its ability to induce an IRF response in KO-IRF3 cells when complexed with LyoVec™ or LTX. On the contrary, the IRF response is unexpectedly increased when using the highly potent agonist 3p-hpRNA complexed to LyoVec™ or LTX. Surprisingly, with either agonist, the NF-κB response is barely affected. These data suggest that the use of different agonists and transfection reagents could highlight overlapping RNA-sensing or regulatory mechanisms.
Back to the top