ASC Knockout & Knockdown Monocytes
| Product | Unit size | Cat. code | Docs. | Qty. | Price | |
|---|---|---|---|---|---|---|
|
THP1-KO-ASC Cells ASC Knockout in THP-1 cells (human monocytes) |
Show product |
3-7 x 10e6 cells |
thp-koascz
|
|
||
|
THP1-KO-ASC vial Additional cell vial |
Show product |
3-7 x 10e6 cells |
thp-koascz-av
|
|||
|
THP1-defASC Cells ASC Knockdown in THP-1 cells (human monocytes) |
Show product |
3-7 x 10e6 cells |
thp-dasc
|
|
||
|
THP1-defASC vial Additional cell vial |
Show product |
3-7 x 10e6 cells |
thp-dasc-av
|
Notification: Reference #thp-koascz-av can only be ordered together with reference #thp-koascz.
Reference #thp-dasc-av can only be ordered together with reference #thp-dasc.
ASC knockout or knockdown in THP-1 cells
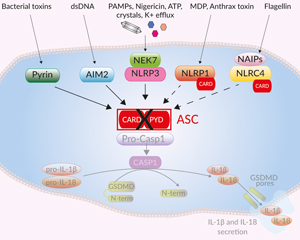
Canonical inflammasome signaling in THP1-KO-ASC cells
ASC (apoptosis-associated speck-like protein containing a CARD domain, also known as PYCARD) is a protein adaptor important in canonical inflammasome responses [1]. ASC's bipartite composition, consisting of one PYD and one CARD domain, allows the recruitment of the CARD-containing pro-caspase-1 to canonical inflammasome sensors that do not contain a CARD domain, such as NLRP3, AIM2, and Pyrin [1].
To foster research on the ASC adaptor, InvivoGen provides cellular tools that have been generated from the human monocytic THP1 cell line through either a stable knockout or knockdown of the ASC gene. THP-1 cells are widely used for inflammasome studies due to their high expression levels of NLRP3, ASC, and pro-caspase-1.
• THP1-KO-ASC cells – Knockout (KO) of the ASC gene
• THP1-defASC cells – Knockdown (KD) of the ASC gene
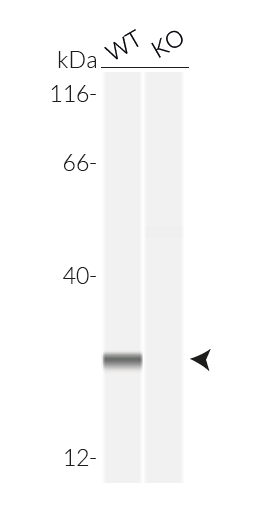
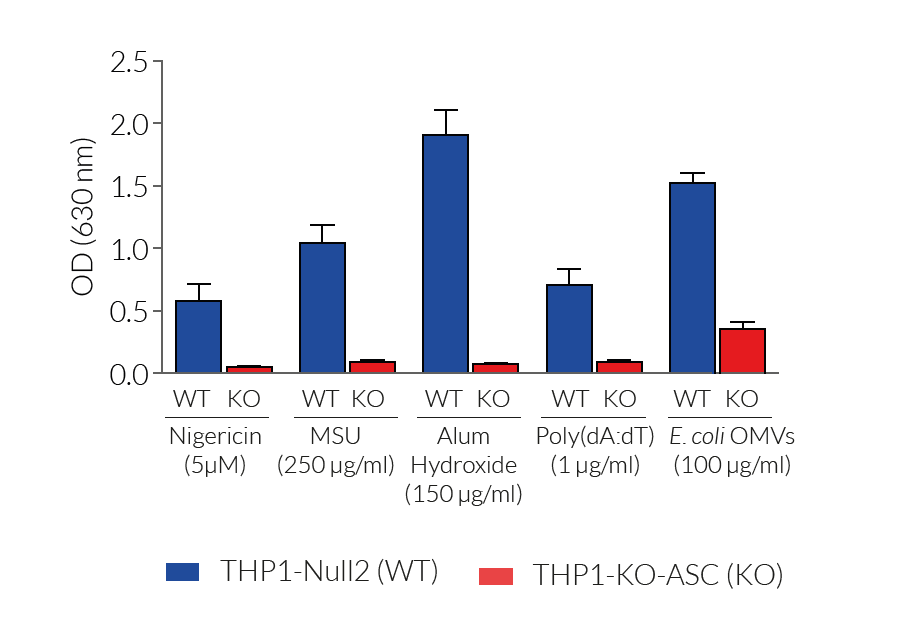
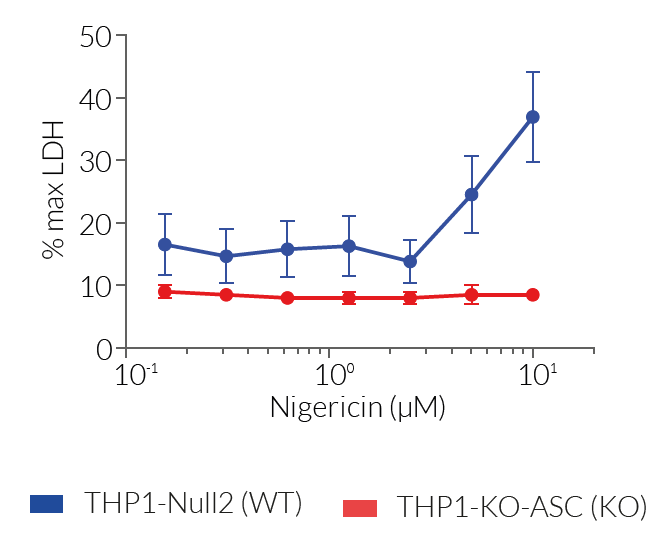
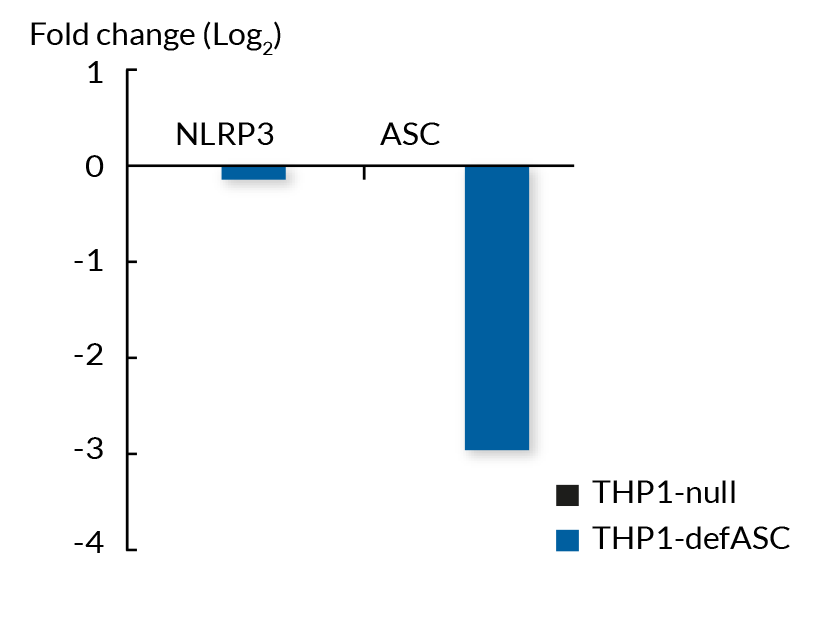
These cell lines exhibit a similar phenotype but have dramatically different genotypes. They share the common phenotype of abrogation of mature IL-1β secretion. However, THP1- defASC cells exhibit a knockdown of ASC gene expression whereas THP1-KO-ASC cells possess a KO of the ASC gene. Indeed, THP1- defASC cells have a maximum 3-fold reduction in ASC expression (RT-qPCR), whereas THP1-KO-ASC cells have no ASC protein expression (Western blot). These cell lines are useful tools in the study of the ASC-dependent inflammasome responses and can also be used as control cell lines for the screening of novel therapeutics that target the ASC signaling pathways.
Features of THP1-KO-ASC cells:
- Generated from the parental cell line THP1-Null2
- Verified biallelic KO of the ASC gene (sequencing, PCR, and Western blot)
- Complete abrogation of mature IL-1β secretion upon activation of the canonical or non-canonical inflammasomes
Features of THP1-defASC cells:
- Verified KD of the ASC gene (RT-qPCR)
- Significantly reduced mature IL-1β secretion upon activation of the canonical inflammasome
- Highly referenced in inflammasome-related literature (see citations)
InvivoGen also provides THP1-ASC-GFP reporter cells that express an inducible ASC::GFP fusion protein to monitor the priming and the activation steps of ASC-dependent inflammasomes using time-lapse confocal or high-resolution fluorescence microscopy.
For detecting and quantifying the release of mature human (h)IL-1β, InvivoGen provides HEK-Blue™ IL-1β sensor cells, which express an NF-κB-inducible SEAP reporter gene. QUANTI-Blue™ Solution allows rapid colorimetric detection and measure of SEAP activity by reading the optical density at 630-650 nm.
For detecting and quantifying pyroptotic cell death, InvivoGen provides THP1-HMGB1-Lucia™ cells, which express a cytoplasmic HMGB1::Lucia luciferase fusion protein that is released in the supernatant upon pyroptosis. The activity of the Lucia luciferase reporter protein can be readily assessed using the QUANTI-Luc™ detection reagent.
![]() Download our Practical guide on Inflammasomes
Download our Practical guide on Inflammasomes
Reference:
1. Mathur A. et al., 2017. Molecular mechanisms of inflammasome signaling. J. Leuk. Biol. 103:233.
Back to the topSpecifications
THP1-KO-ASC cells
Antibiotic resistance: Zeocin®
Growth medium: RPMI 1640, 2 mM L-glutamine, 25 mM HEPES, 10% (v/v) heat-inactivated fetal bovine serum (FBS), 100 U/ml penicillin, 100 µg/ml streptomycin, 100 µg/ml Normocin™
Quality Control:
- Biallelic ASC knockout has been verified by PCR, DNA sequencing, Western blot (WES™), and functional assays.
- The stability for 20 passages, following thawing, has been verified.
- These cells are guaranteed mycoplasma-free.
THP1-defASC cells
Antibiotic resistance: Hygromycin B
Growth Medium: RPMI 1640, 2 mM L-glutamine, 25 mM HEPES, 10% (v/v) heat-inactivated fetal bovine serum (FBS), 100 U/ml penicillin, 100 µg/ml streptomycin, 100 μg/ml Normocin™
Quality control:
- ASC deficiency (def) has been verified by qRT-PCR and functional assays.
- The stability for 20 passages, following thawing, has been verified.
- These cells are guaranteed mycoplasma-free.
Contents
THP1-KO-ASC cells
- 3-7 x 106 THP1-KO-ASC cells in a cryovial or shipping flask
- 1 ml of Zeocin® (100 mg/ml). Store at 4 °C or at -20 °C.
- 1 ml of Normocin™ (50 mg/ml). Normocin™ is a formulation of three antibiotics active against mycoplasmas, bacteria, and fungi.
![]() Shipped on dry ice (Europe, USA & Canada)
Shipped on dry ice (Europe, USA & Canada)
THP1-defASC cells
- 3-7 x 106 THP1-defASC cells in a cryovial or shipping flask
- 1 ml of Hygromycin B Gold (100 mg/ml)
- 1 ml of Normocin™ (50 mg/ml). Normocin™ is a formulation of three antibiotics active against mycoplasmas, bacteria, and fungi.
![]() Shipped on dry ice (Europe, USA, Canada and some areas in Asia)
Shipped on dry ice (Europe, USA, Canada and some areas in Asia)
Details
Inflammasomes are multimeric protein complexes that are crucial for host defense against infection and response to endogenous danger signals. The canonical inflammasome response is driven by aggregation of a sensor (i.e. NLRP3) with the ASC adaptor and pro-caspase-1. Activation of caspase-1 (CASP1) induces the maturation of pro-IL-1β/pro-IL-18 and cleavage of the pore-forming protein gasdermin D (GSDMD), leading to secretion of IL-1β/ 18 and pyroptosis.
ASC is essential to canonical inflammasome sensors that do not contain a CARD domain, such as NLRP3, AIM2, and Pyrin [1]. ASC's bipartite composition, consisting of one PYD and one CARD domain, allows the recruitment of the CARD-containing pro-caspase-1 to these sensors. The NLRP1 and NLRC4 inflammasome sensors have a CARD domain, and can thus recruit pro-caspase-1 either directly, or through ASC. However, NLRP1 or NLRC4 activation in the absence of ASC triggers a reduced secretion of mature IL-1β and IL-18 [1]. Importantly, non-canonical inflammasomes (i.e. CASP4/5/11) activate CASP1 indirectly: their activation triggers GSDMD-driven release of alarmins and K+ efflux, which in turn, induce NLRP3- and CASP1-mediated IL-1β/-18 maturation and secretion. Therefore, the absence of ASC affects the downstream inflammatory signaling from the non-canonical inflammasome also.
In resting cells, ASC is present in a soluble and diffuse form both in the cytoplasm and nucleus [2]. Inflammasome activation in most cells leads to the formation of one large, micrometer-sized, ASC ‘speck’ per cell, thus concentrating CASP1 activation sites [2,3]. Yet, the number of ASC specks may dependent on the inflammasome inducer used. For example, Nigericine promotes the formation of numerous specks, whereas ATP leads to the accumulation of fewer specks [4].
1. Mathur A. et al., 2017. Molecular mechanisms of inflammasome signaling. J. Leuk. Biol. 103:233.
2. Hoss F. et al., 2017. Assembly and regulation of ASC specks. Cell. Mol. Life Sci. 74:1211.
3. Stutz A. et al., 2013. ASC speck formation as a readout for inflammasome activation. Methods Mol. Biol.
4. Zha Q. et al., 2016. ATP-induced inflammasome activation and pyroptosis is regulated by AMP-activated protein kinase in macrophages. Front Immunol. 7:597.