Human TLR7 Reporter THP-1 Cells
| Product | Unit size | Cat. code | Docs. | Qty. | Price | |
|---|---|---|---|---|---|---|
|
THP1-Dual™ hTLR7 Cells Human TLR7 Overexpressing - THP-1 Reporter Monocytes |
Show product |
3-7 x 10e6 cells |
thpd-htlr7
|
|
||
|
THP1-Dual™ hTLR7 vial Additional cell vial |
Show product |
3-7 x 10e6 cells |
thpd-htlr7-av
|
|||
|
THP1-Dual™ hTLR7 KO-TLR8 Cells Human TLR8 Knockout and TLR7 Overexpressing - THP-1 Reporter Monocytes |
Show product |
3-7 x 10e6 cells |
thpd-htlr7-ko8
|
|
||
|
THP1-Dual™ hTLR7 KO-TLR8 vial Additional cell vial |
Show product |
3-7 x 10e6 cells |
thpd-htlr7-ko8-av
|
Notification: Reference #thpd-htlr7-av can only be ordered together with reference #thpd-htlr7.
Reference #thpd-htlr7-ko8-av can only be ordered together with reference #thpd-htlr7-ko8.
TLR7 expressing reporter monocytes
InvivoGen expands its collection of human monocytic reporter cell lines designed to monitor the intertwined NF-κB and IRF responses of the Toll-Like Receptor 7 (TLR7).
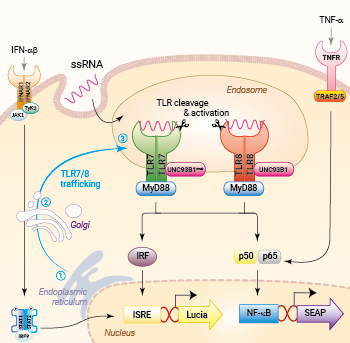
Trafficking and signaling in THP1-Dual™ TLR7/8 cells
THP1-Dual™ hTLR7 cells and THP1-Dual™ hTLR7 KO-TLR8 cells were generated from the THP1-Dual™ cell line harboring two inducible reporter genes, allowing the simultaneous monitoring of the NF-κB and IRF pathway, by monitoring the SEAP and Lucia luciferase activities, respectively. They feature a stable expression of the human TLR7 ± a knockout (KO) of the endogenous TLR8 gene. To enable TLR7 signaling, a mutated (mut) version of the TLR-trafficking protein UNC93B1 is also co-expressed.
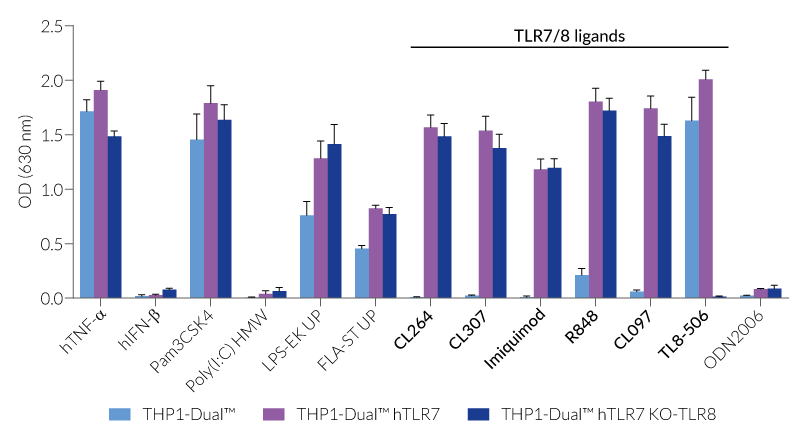
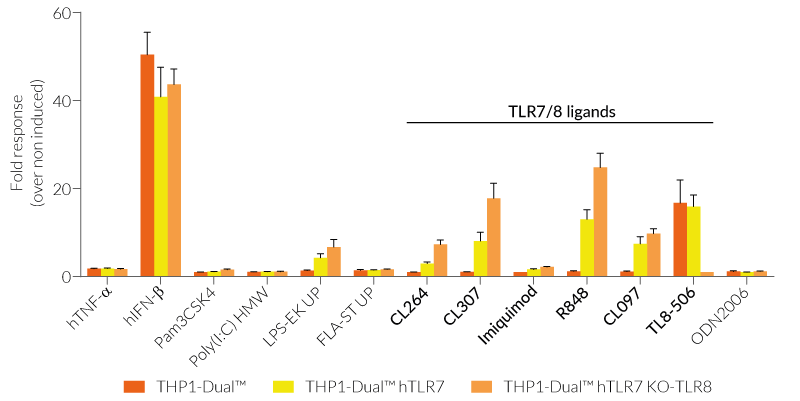
These cells are derived from human leukemia monocytic THP-1 cells, a common model to study monocyte/macrophage functions, mechanisms, and signaling pathways. They express several pattern recognition receptors (PRRs) and co-adaptors, including TLR8 and UNC93B1, but not TLR7 [1-2]. Indeed, THP1-Dual™ cells do not respond to TLR7-specific ligands (see figures).
Only the co-expression of TLR7 with its chaperone protein UNC93B1mut makes them responsive to TLR7-specific ligands (see figures).
By additionally introducing a KO of endogenous TLR8, THP1-Dual™ hTLR7 KO-TLR8 cells do not respond to TLR8-specific ligands (see figures). Interestingly, this KO increases the IRF response to various TLR7/8 ligands in these cells, when compared with THP1-Dual™ hTLR7 cells (see figures). In accordance with these results, previous studies have demonstrated that TLR8 has an inhibitory effect on TLR7 when both are co-expressed [3]. Therefore, a TLR8 KO abolishes this inhibition, leading to a higher TLR7 activation (see figures).
Both TLR7 and TLR8 recognize viral single-stranded RNA (ssRNA) as well as small synthetic molecules. Despite their structural homology and similar signaling pathway, TLR7 and TLR8 display different ligand specificities and tissue expression patterns [4].
Key Features:
- Stable co-expression of human (h)TLR7 and UNC93B1mut
- Verified biallelic KO of the hTLR8 gene
- Restored response to TLR7-specific ligands
- No response to TLR8-specific ligands
- Distinct monitoring of NF-κB or IRF activation by assessing the SEAP and Lucia luciferase activities
Applications:
- Defining the distinct role of TLR7 and TLR8 in the IRF and NF-κB-dependent pathway
- Highlighting possible overlap between TLR7 and TLR8 signaling pathways
- Screening for TLR7- and TLR8-specific agonists or inhibitors
![]() Read our review on TLR7 and TLR8: same, same... but different!
Read our review on TLR7 and TLR8: same, same... but different!
References:
1. Hornung V. et al. 2002. Quantitative expression of toll-like receptor 1-10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J Immunol.; 168(9):4531-7.
2. Majer, O., et al. 2019. UNC93B1 recruits syntenin-1 to dampen TLR7 signalling and prevent autoimmunity. Nature 575, 366–370.
3. Wang J, et al., 2006. The functional effects of physical interactions among Toll-like receptors 7, 8, and 9. J Biol Chem.; 281(49):37427-34.
4. Georg P. & Sander L.E., 2019. Innate sensors that regulate vaccine responses. Curr. Op. Immunol. 59:31.
Specifications
Growth medium: RPMI 1640, 2 mM L-glutamine, 25 mM HEPES, 10% (v/v) fetal bovine serum (FBS), 100 U/ml penicillin, 100 µg/ml streptomycin, 100 µg/ml Normocin™
Antibiotic resistance: Blasticidin and Zeocin®
Quality Control:
- Human TLR7 and UNC93B1mut overexpression has been verified by RT-qPCR and functional assays.
- Biallelic TLR8 knockout has been verified by PCR, DNA sequencing, Western blot, and functional assays.
- The stability for 20 passages, following thawing, has been verified.
- These cells are guaranteed mycoplasma-free.
Contents
Please note: Each cell line is sold separately. See TDS for the exact contents of each cell line.
- 3-7 x 106 THP1-Dual™ hTLR7 cells OR THP1-Dual™ hTLR7 KO-TLR8 cells in a cryovial or shipping flask
- 1 ml of Normocin™ (50 mg/ml). Normocin™ is a formulation of three antibiotics active against mycoplasmas, bacteria, and fungi.
- 1 ml of Zeocin® (100 mg/ml)
- 1 ml of Blasticidin (10 mg/ml)
- 1 tube of QUANTI-Luc™ 4 Reagent, a Lucia luciferase detection reagent (sufficient to prepare 25 ml)
- 1 ml of QB reagent and 1 ml of QB buffer (sufficient to prepare 100 ml of QUANTI-Blue™ Solution, a SEAP detection reagent)
![]() Shipped on dry ice (Europe, USA, Canada, and some areas in Asia)
Shipped on dry ice (Europe, USA, Canada, and some areas in Asia)
FAQ Cell Lines
 Any questions about our cell lines ? Visit our frequently asked questions page
Any questions about our cell lines ? Visit our frequently asked questions page
Back to the top
Details
Toll-Like Receptors 7 and 8
In humans, four Toll-Like Receptor (TLR) family members TLR3, TLR7, TLR8, and TLR9, mainly found in the endosome, are specialized in sensing viral-derived components. TLR7 and TLR8 recognize single-stranded (ss)RNA structures, such as viral ssRNA, miRNA, and various synthetic agonists [1]. Despite their similarities in PAMP (pathogen-associated molecular pattern) recognition, structure, and signaling partners, they highly differ in expression profiles and signaling responses, with TLR7 being more involved in the antiviral immune response and TLR8 mastering the production of proinflammatory cytokines [2]. TLR7 is mainly found in plasmacytoid dendritic cells (pDCs) and B cells, whereas TLR8 is highly expressed in monocytes, monocyte-derived DCs (mDCs), and macrophages [3].
TLR7 and TLR8 trafficking and signaling
Upon viral infection, the chaperone protein UNC93B1 interacts with TLR7 and TLR8, thereby facilitating their trafficking from the endoplasmic reticulum via the Golgi into the endosomes [4]. Subsequently, TLR7 and TLR8 undergo proteolytic cleavage and dimer rearrangement [1,3]. While TLR7 dimerizes upon ligand binding, TLR8, which exists as an unliganded inactive dimer, performs structural reorganization after ligand recognition [5]. Once activated, both TLR7 and TLR8 recruit the adaptor protein MyD88 to trigger IRF, AP-1, and NF-κB responses via TRAF6 (TNF receptor-associated factor 6) [1,3]. Depending on the stimulus and cell type, TLR7-mediated signaling induces IFN-α and IFN-regulated cytokines or T helper 17 (Th17) polarizing cytokines, such as interleukin (IL)-1β and IL-23, whereas Th1-type cytokine (IL-12) production depends on TLR8 [6].
TLR7 and TLR8 therapeutic targeting
The involvement of nucleic acid-sensing mechanisms in the immune response against infections and other diseases makes them interesting targets for drug design [6]. TLR7/8 agonists are currently been tested as vaccine adjuvants and immunomodulatory therapeutics. They are extensively studied in the context of viral infection (e.g. SARS-CoV-2, Influenza, HIV), autoimmune (e.g. asthma, Lupus), and autoinflammatory diseases (e.g. cancer) [1-6]. Understanding the fundamental differences between these two related receptors could potentially be harnessed to discover novel drugs and improve vaccine efficacy/safety [6].
References:
1. Martínez-Espinoza I & Guerrero-Plata A. 2022. The Relevance of TLR8 in Viral Infections. Pathogens. 11(2):134.
2. Salvi V, et al., 2021. SARS-CoV-2-associated ssRNAs activate inflammation and immunity via TLR7/8. JCI Insight.;6(18):e150542.
3. Georg P. & Sander L.E., 2019. Innate sensors that regulate vaccine responses. Curr. Op. Immunol. 59:31.
4. Majer, O., et al. 2019. UNC93B1 recruits syntenin-1 to dampen TLR7 signalling and prevent autoimmunity. Nature 575, 366–370.
5. Asami J, Shimizu T. 2021. Structural and functional understanding of the toll-like receptors. Protein Sci. (4):761-772.
6. de Marcken M, et al., 2019. TLR7 and TLR8 activate distinct pathways in monocytes during RNA virus infection. Sci Signal.;12(605):eaaw1347.